DRYING FLEXIBLE ENDOSCOPES: FACT AND FICTION
Overview
Guide to Study
eLearning Activity
Flexible endoscopes are recognized as one of the most difficult medical devices to clean and decontaminate for safe patient reuse. More than 50 million flexible endoscopic procedures are performed each year. These medical devices allow endoscopists to provide diagnostic and therapeutic procedures for the gastrointestinal tract using minimally invasive techniques that ensure the patients spend the least possible amount of time in the healthcare facility. The downside to these complex medical devices is the lack of ease and consistency in ensuring the scope is safe for use the next time it is needed.
The cleaning, decontamination, drying and storage of scopes is an arduous process that is repeated over and over during working hours by nurses and technicians. Even though the steps are repetitive, the scopes are not all the same and meticulous attention to detail is required when following the manufacturer’s instructions for use (IFU). Inconsistencies in reprocessing have been blamed for microbial exposures and infection transmissions during the use of flexible endoscopes for endoscopic procedures. Human error has been identified as the major contributing factor for lapses in endoscope reprocessing.
This program will focus on three of the critical touch points in endoscope reprocessing: drying, storage and clean transport. The Society for Gastroenterology Nurses and Associates (SGNA), the Association of periOperative Registered Nurses (AORN) and the Association for the Advancement of Medical Instrumentation (AAMI) all suggest drying is as critical to the prevention of microbial growth as is cleaning or the removal of as much bioburden as possible before the scope is high level disinfected. We will discuss strategies for improving drying and storage of flexible scopes to reduce microbial growth and meet society guidelines for endoscope drying and storage.
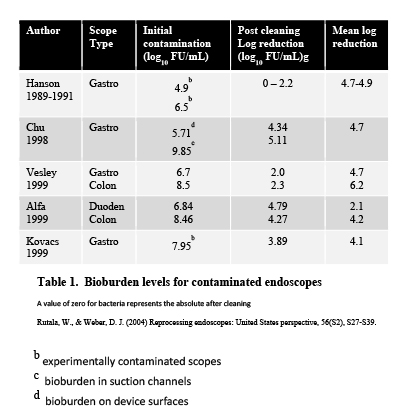
Numerous studies have focused on the contamination level of flexible endoscopes following a procedure. Results showed contamination levels range from 5 log10 to 10 log10 of bioburden, or 100,000 CFU/cm2 to 10 billion CFU/cm2
(Table 1). Gastroscopes had the highest contamination levels while colonoscopes were somewhat less. Bronchoscopes were not included in these studies. The importance of this high level of contamination becomes evident when we understand the work Alfa
et al. (1999) did in identifying the amount of bioburden that is removed with cleaning. She and her team demonstrated that cleaning could reduce the level of bioburden by five to tenfold. This cleaning activity includes initial point-of-use pretreatment along with manual or automated cleaning. Once cleaning has occurred there may still be remaining viable microorganisms, but the level of microbial loading has decreased sufficiently to allow successful high-level disinfection. (High level disinfectants must be able to kill at a minimum 5 logs10 before they can be cleared by the Food and Drug Administration; decreasing the bioburden level to 3-4 logs makes it much easier to kill the remaining microbial load.) By completing these three decontamination steps, you should be able to prepare a safe flexible endoscope for the next patient use. By ensuring that flexible endoscopes are subsequently dried, handled properly and protected in storage and in transport, you will be able to sustain the safety of your high-level disinfected endoscopes.
Drying is Step 8 in the Endoscope Processing Cycle. It can be done manually, or it can be done automatically in a validated drying cabinet.
Manual Drying
After the scope has been cleaned, inspected, and high level disinfected, it needs to be rinsed with critical water and purged with alcohol. The alcohol is pushed through each of the channels. Next, the channels need to be injected with low pressure instrument grade forced air. The exterior surfaces are then dried using a low lint or lint free cloth. Do not use a syringe to push room air through the channels; the syringe is not powerful enough and the air is not sufficiently free of contamination to effectively dry the channel.
Automated Drying
If your AER has an alcohol purge and air purge cycle that follows the final rinse cycle, it is optimal to perform those steps in the AER. If your AER does not have these cycles, the alcohol purge would be done manually, as described above. The alcohol purge is intended to achieve two goals: displace water and bind with the remaining water to facilitate drying, since alcohol dries more quickly than water. The alcohol purge and any automated air purge do not dry the scope! They are akin to the “spin cycle” in a washing machine, where excess moisture is removed before the laundry is placed in a clothes dryer.
All society guidelines recommend using low pressure forced air for drying channels of the endoscope. Instrument grade air is preferred. Instrument grade air meets specifications for compression level, filtering of particulates, concentration of oil and level of dryness. (There is a difference in delivery between medical grade air and instrument grade air. Although the quality is the same, air deemed “medical grade” is for patient use only. It is not plumbed unless it is intended for patient use in patient care units. Medical grade air should not be used for drying medical devices because regulatory agencies mandate there can be no possibility for error or contamination from other sources when using medical grade air for patients. Exception: If specific backflow valves are in place or the Endoscopy Suite air delivery system is entirely separate from the patient air delivery system, medical grade air may be used. Best practice would suggest using a separate source for instrument grade air.)
Instrument grade air has been used extensively in Sterile Processing Departments for many years. It may be stored in a tank or it may be connected directly to a facility source. When drying flexible endoscopes, the instrument grade air is injected into the endoscope channel using a purpose-built connecting device so that the air can flow evenly and consistently across all internal surfaces. Instrument grade air delivered by a validated drying cabinet provides a low-pressure stream of air so as not to damage instruments.
Managed flow of air is recommended by all guidelines; unregulated “pistols” may use so much force that they damage channels, while syringes used to inject air through the channels are not strong enough to force the residual fluid out of the channels. All professional society guidelines recommend avoiding the use of syringes for channel drying.
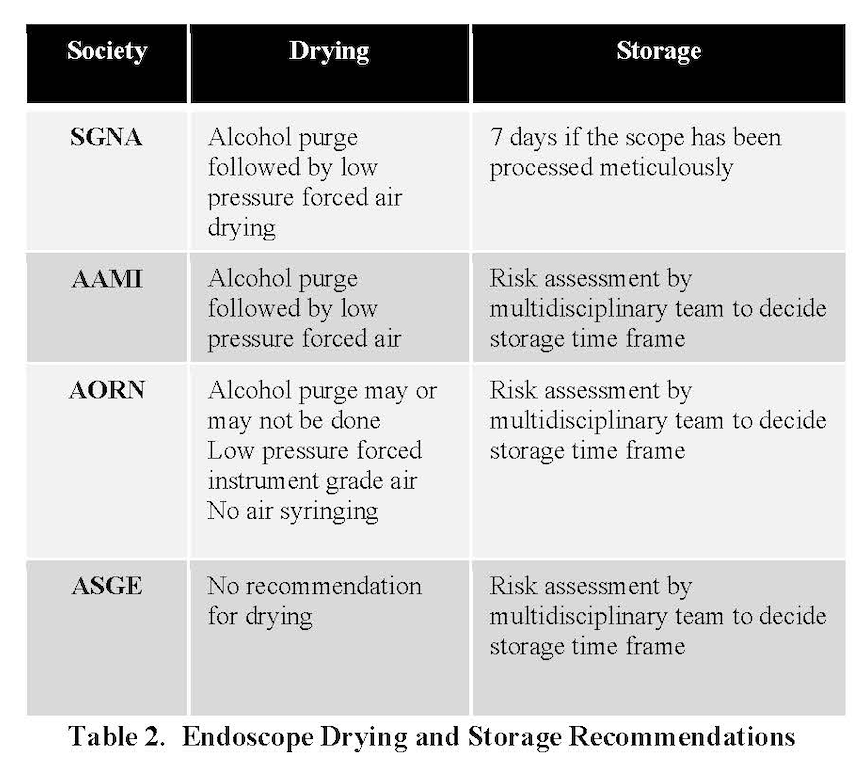
The professional society guidelines that are typically used in endoscope reprocessing and drying are listed in Table 2. SGNA suggests an alcohol purge followed by low pressure forced air drying, with external wipe down of the scope. AORN suggests an alcohol purge may be used. AAMI suggests an alcohol purge followed by low pressure forced air. The American Society of Gastrointestinal Endoscopy (ASGE) does not make any recommendations about drying.
Researchers have been increasingly studying the importance of drying to help improve patient safety. It is well documented that scopes can be heavily soiled following a procedure, and meticulous attention is paid to cleaning and high-level disinfecting them in order to make them safe. (As an example, Alfa, Degagne and Olson (1999) looked at initial loading of bioburden in colonoscopes, gastroscopes and bronchoscopes. Their research showed bioburden loading to be between 5 logs and 10 logs of contamination. They also discovered cleaning could reduce levels of protein, endotoxin and sodium ion levels between five and tenfold for all these endoscopes, while carbohydrates were below detectable levels. Viable bioburden was reduced 3-5 logs. This reduction is significant, since high-level disinfectants can kill up to 5 logs of bacterial bioburden. Remember, if cleaning can remove the majority of bacterial bioburden and high-level disinfectants kill the remaining bacterial load, the scope is then safe to be used on the next patient when it comes out of the high-level disinfectant.)
In the late 1990s, healthcare providers were unaware of the serious risks of recontamination following high-level disinfection. Scopes were routinely “hung to dry,” in anticipation that gravity and evaporation would render the scope dry. It was known then that drying provides an environment that can prevent and eliminate any remaining viable microorganisms from reproducing, and endoscopy professionals have always had that goal in mind when processing their equipment.
What was not known was the level of recontamination that occurs when scopes are stored, transported and otherwise handled when they are not completely dry. Several studies since that time have identified drying as a step equally important to cleaning in making flexible endoscopes safe for patient use.
Storing a scope when it is not completely dry is an invitation for residual or airborne microorganisms to make themselves at home on the endoscope; based on how quickly bacteria replicate, 500 bacteria can become 60 million bacteria within eight hours. A damp scope with just a little bit of bacteria landing on it could be colonized over the span of just one weekend. Most research showed scopes could be contaminated exogenously, or externally, through improper handling, not wearing clean personal protective equipment, environmental contamination, mishandling of the scope or transport contamination. Rejchrt et al. (2004) and Brock et al. (2015) discovered microbial growth over periods of time if the scope was not dried sufficiently and handled correctly. Pineau (2008) showed little microbial growth occurred if ten minutes of low pressure forced air drying was used instead of the two-minute air-purge cycle that is integrated into most automated endoscope reprocessors. While cleaning removes contamination, drying prevents microbial growth. Pajkos et al. (2013) showed that biofilm forms on scopes that are not completely dry, making it even more difficult to process a scope successfully and keep it patient ready and safe for use.
Ofstead (2016) published a study showing residual fluid was present in channels of scopes that had been fully reprocessed. Even though the scopes had been hung vertically to “drip dry”, borescope inspection documented through the taking of digital pictures that there were still fluid droplets in the channels of 19 out of 20 endoscopes.
Barakat et al. in 2019 confirmed that significantly more fluid droplets were evident in flexible endoscopes that were manually dried using forced filtered air delivered through an air gun compared with drying that was done with an automated device that used forced filtered air. Another study published in 2019,
conducted by Perumpail et al., compared the drying effectiveness of an automated drying/storage cabinet with a conventional storage cabinet. Their analysis showed that internal channels in the automated system were dry after one hour, while there was residual fluid in internal channels of the endoscopes store in a conventional cabinet. Both these studies conclude that drying is an important aspect of reducing microbial growth and contamination after high-level disinfection has been completed.
Many factors contribute to bacterial growth in flexible endoscopes after they have been high-level disinfected. Scopes can become contaminated from a variety sources: bacteria from the air and water (including contaminated rinse water) and microorganisms transmitted through contact with bare hands are two frequent sources. Unfortunately, undetected micro cracks and scratches in or on the endoscope can harbor microorganisms even when IFUs for decontamination and disinfection were followed carefully; those microbes can reproduce once the damp endoscope is put into storage.
Microbes normally require a moist environment to reproduce. When water is present and the conditions are right, bacteria like E. coli and Clostridium perfringens have the ability to reproduce every 10-20 minutes. Another significant risk associated with constant moisture is biofilm formation; provided a source of moisture, biofilm can quickly colonize a scope and provide cover or shelter for viable organisms, protecting them from destruction during subsequent cleaning and high-level disinfection.
Perumpail et al. (2019) demonstrated in a controlled study that 500 CFU of bacteria introduced into high-level disinfected endoscopes can grow in just 24 hours to levels ranging from 8.1 million CFUs/cm2 on bronchoscopes to 70 million CFUs/cm2 on duodenoscopes if there is no drying of internal channels. Conversely, in endoscopes where internal channels were dried in a validated drying cabinet with a continual flow of low pressure filtered air, the internal channels showed no growth; the drying of the surfaces created an inhospitable environment for the
bacteria and they died. The impact of just a small amount of contamination should not be underestimated.
Pineau, Villard, Duc & Marchetti (2008) showed that when using a drying cabinet, residual microbial counts could be decreased and there was no evidence of microbial growth. At the same time, vertical hanging of scopes in a conventional cabinet showed a stable or even increased microbial level during storage, since microbial reproduction can occur every 20 minutes.
Foxcroft, Hautefeuille, Marchetti, Pineau, and Laugier (2013) showed that protected storage environments help control microbial growth. They also discovered that drying cabinets produced less environmental contamination within the drying storage cabinet compared to the environment in the conventional storage cabinet. These cabinets were dedicated for endoscope drying and storage.
Another important aspect of maintaining and protecting flexible endoscopes are the cabinets where they are stored. All society guidelines recommend a protected storage area for patient ready endoscopes. This can be cabinets that lock or in a room with limited access. AORN and AAMI do not recommend storage of scopes in a procedure room. SGNA stipulates that all reprocessing activities, including storage, should be in a designated, dedicated area that is separate from where procedures are performed. They also noted that storage should be in a clean, well-ventilated and dust-free area in order to keep scopes dry and free of contamination.
Until recently several professional nursing societies recommended specific “hang time” or storage times for flexible scopes if they had been processed completely. Schmelzer and Daniels (2016) researched literature to determine if there was sufficient evidence to warrant a recommendation for storage time. Their findings persuaded them to recommend 7 days for storage if the flexible endoscope has been meticulously reprocessed following the manufacturer’s instructions for use (see Table 2).
Currently only SGNA recommends a storage time of 7 days if the scope manufacturer’s instructions for use have been followed meticulously during reprocessing. AORN and AAMI no longer specify a time frame for storage before a scope must be reprocessed. Both organizations recommend a multidisciplinary group made up of staff within the healthcare facility who are knowledgeable about flexible endoscopes could review evidence-based research and come to a consensus on length of storage time for flexible endoscopes before they must be reprocessed. There is sufficient research on drying and storage for a multidisciplinary team to assess whether a longer or shorter period of time will work for their facility.
Table 3 provides examples of researchers that reviewed evidence and made recommendations about acceptable storage time. The results are widely different; these differences suggest there may be cultural disparities as well as different ways of assessing “patient readiness”.
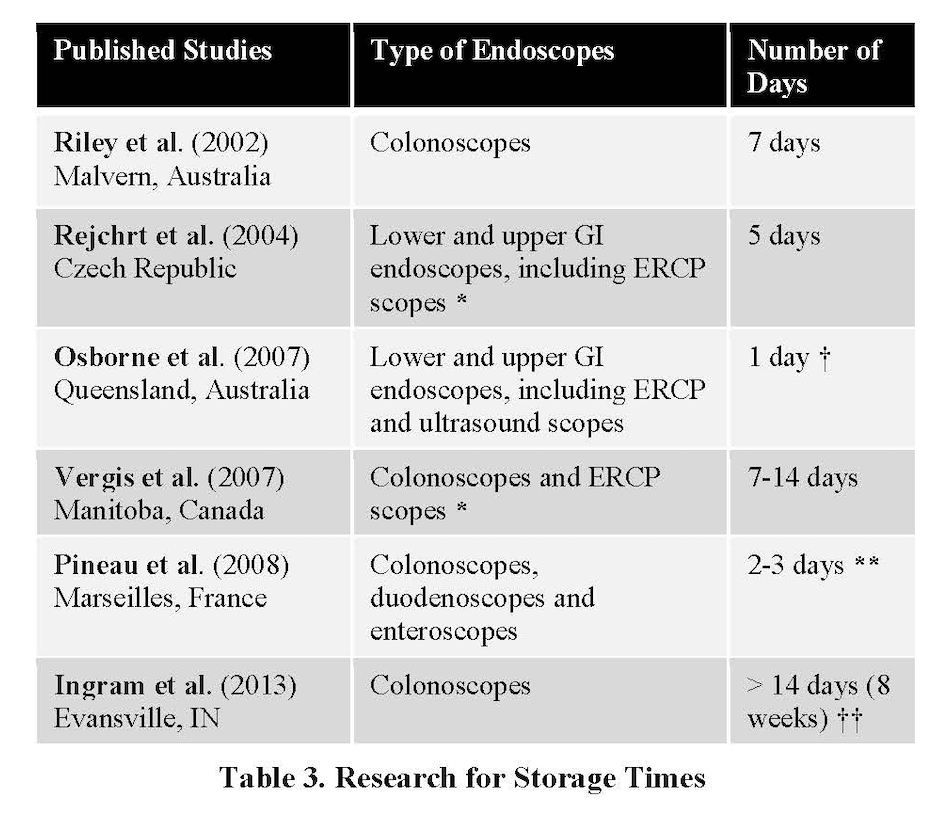
The collective evidence regarding the maximum safe storage time for processed endoscopes is inconclusive. Recommendations from professional organizations for maximum storage times for flexible endoscopes are not in agreement; recommended storage times range from three hours to one month.
Recommendations from studies show a wide range of acceptable storage times; results from safe storage studies when flexible endoscopes are correctly processed range from 48 hours to 56 days after processing. A study by Perumpail et al. (2019) showed no microbial growth at 31 days for endoscopes that were stored in a validated drying cabinet with channel connectivity.
Safe storage times may be affected by variables that are unique to the facility, including the type of endoscopes processed and stored, processing effectiveness, storage conditions, compliance with manufacturers' IFU, frequency of endoscope use, and patient population. There are benefits to eliminating unnecessary processing of flexible endoscopes that include reduced processing costs, reduced wear and tear on the endoscope and processing equipment, and lower replacement and repair costs.
Whatever the time frame selected, it should be incorporated into the facility’s policy and procedure.
As noted in section III, Drying Flexible Endoscopes, the major difference between validated drying cabinets and storage cabinets is the drying cabinet’s ability to flow filtered air through an endoscope’s channels. Because of their continual positive air flow, drying cabinets provide a positive pressure environment when the cabinet door is opened, preventing contamination from entering the storage area.
AORN has identified different categories of cabinets used for endoscope storage. Their recommendation for optimal practice is a drying cabinet that has a validated drying cycle that uses filtered, low pressure air with channel connectivity for each individual endoscope. These drying cabinets can use horizontal placement for drying and storage or use conventional vertical hanging.
Current AAMI, AORN and SGNA guidelines acknowledge an acceptable storage cabinet category for a completely dried scope would be a traditional vertical-hanging cabinet with circulation of either filtered or room air through the cabinet. This category would not include channel connectivity.
The storage cabinet historically seen most often is the ventilated cabinet with hooks for vertical hanging. These cabinets are fairly inexpensive but may present handling challenges. Storage cabinets where the scopes are hung very closely together make it difficult to remove a scope or put one into the cabinet without touching other scopes or the sides of the cabinet. All contact matters when it comes to keeping a scope from becoming contaminated. Unless you dry your scopes in a validated drying cabinet before they are placed into storage, you run the risk of a damp scope being exposed to contamination through inadvertent touch and subsequently growing microbial colonies that are supported by the moist environment of the scope and the cabinet.
It is not unusual to see a storage cabinet with vertical hanging used as a drying cabinet. The theory behind this practice is that gravity and evaporation will remove residual moisture from the scope. Unfortunately, due to the narrow diameter of internal channels, the high surface tension of the channel sheathing, and the humid environment typically found in these cabinets, successful drying is difficult to achieve with this type of cabinet. A ventilated cabinet may only have air openings at the bottom of the cabinet, to evaporate moisture that would otherwise accumulate on the floor. There is insufficient air flow to provide evaporation of internal surfaces.
Regardless of whether a cabinet is designed for storage or for drying and storage, guidelines outline additional recommendations to protect the scopes within. Controlling access to high-level disinfected scopes is paramount in protecting them; it is best to have a mechanism to lock a cabinet and/or the room in which the scopes are stored. Ideally, you would be able to track access, so you’d know when an endoscope had been stored, handled, or removed, and by whom. It would also be helpful to have data documenting the level of dryness that had been achieved and a system to notify staff when a scope needed to be re-processed. (Validated drying cabinets with a data management system have been mandated in many European countries and are increasingly prevalent in North America.)
All researchers suggest that more studies are needed to determine the effectiveness of drying regarding flexible endoscopes. Definitions are needed for terms such as” bone dry” and “ventilation”. Based on these research data, healthcare providers in endoscopy settings may need to rethink how we dry and store flexible endoscopes.
AAMI, AORN & SGNA have all made recommendations about best practices for transport. Most recommendations focus on transporting contaminated scopes, since these present the biggest risk to the largest group of people. All the guidance on contaminated transport says that contaminated scopes should be transported in a closed, rigid, puncture resistant, leak-proof system, and all recommend using a biohazard symbol. This is also a requirement from OSHA so it’s a “must do”. Additionally, all caution the users against “over-coiling” of the endoscope any time it is being transported. All these guidelines reference specific research to support their recommendations. Other recommendations of interest:
- AAMI recommends using a closed transport system for transporting a patient-ready scope from storage to the procedure area. AAMI specifies that any endoscope that has been high-level disinfected needs to be protected from contamination.
- AORN recommends that endoscopes should always be transported in a horizontal position; they should not be suspended vertically.
- SGNA and AAMI recommend using clean gloves for handling of any clean scopes. The rationale for this recommendation: Disinfected endoscopes can become recontaminated by hands and/or communication with surfaces while being handled and transported. Use of a barrier system can prevent recontamination.
Unfortunately, processing activities frequently do not follow guidelines for practice. According to Dirlam Langlay et al. (2013), “Reprocessing lapses are an ongoing and widespread problem despite the existence of guidelines.” Ofstead et al. (2010) discovered that only 45% of employees reported using any forced air drying for high-level disinfected endoscopes, despite the fact that guidelines require drying of all scope surfaces before storage. As with any process where humans are concerned, errors can and do occur. In the endoscopy suite, those errors may have potentially serious consequences.
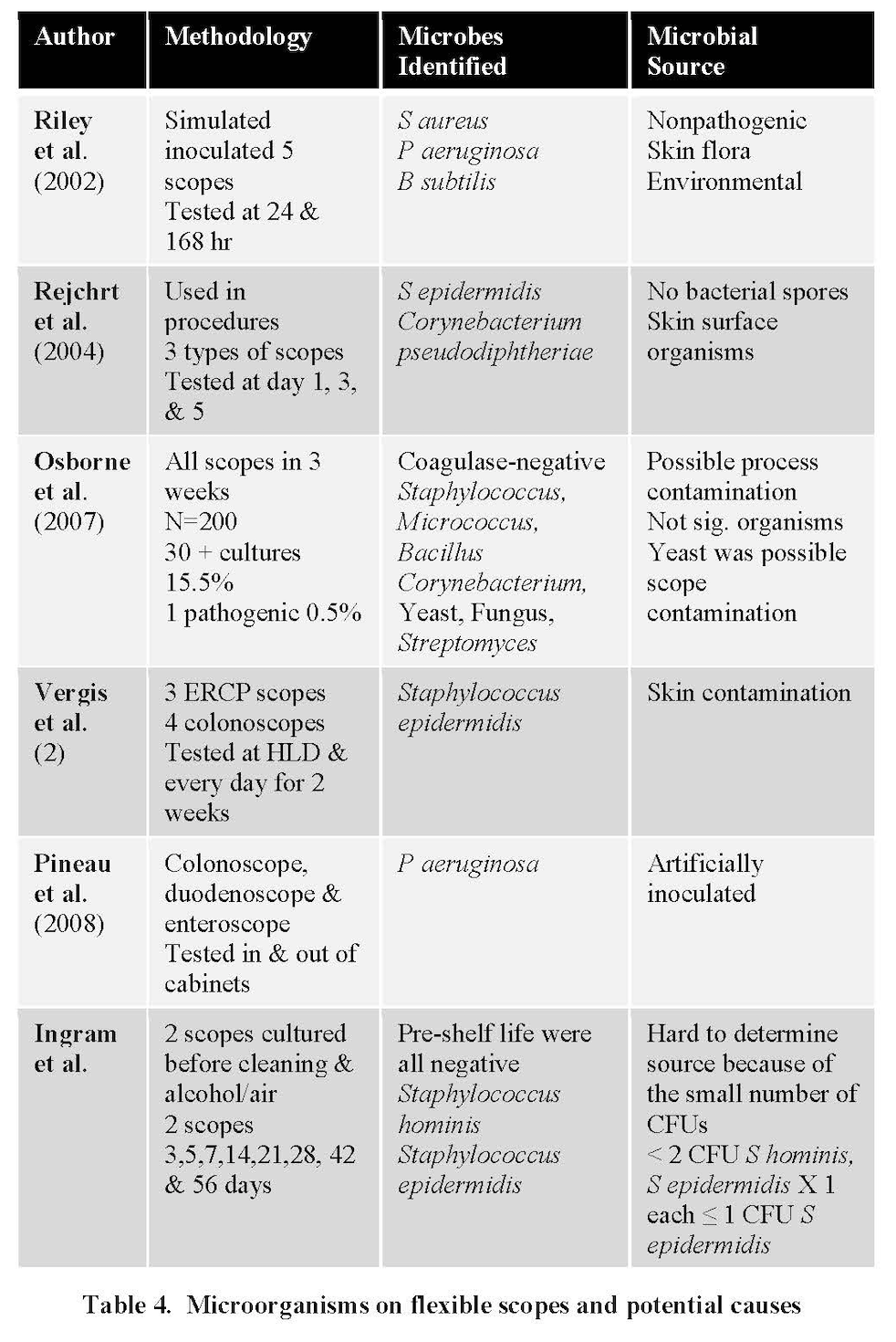
In Table 4, you see a listing of microorganisms found on flexible endoscopes after they had been high-level disinfected and stored. Where did these microorganisms come from? The table lists sources of contamination that have been studied through testing. Over 50% of them show contamination from mishandling. This contamination may be attributable to not wearing gloves or to the scope coming in contact with clothing, contaminated gloves, other scopes or surfaces.
Human Error - Drying
What are the reasons you’d find a wet scope in storage? Possibly, the staff did not perform the manual drying process on the external surfaces. Maybe the scope was stored with the insertion tube looped over and around a hook or placed horizontally without hooking up to a validated connection in a drying cabinet. Most likely, the scope with residual moisture was handled by someone who was unaware of the challenges associated with “drip drying” and the potential risks to patients from storing a scope that was not completely dry when placed into storage. Unless the scope is immediately placed into a validated drying cabinet, its external surfaces must still be dried with a low-lint or lint-free cloth after high-level disinfection; there will be residual moisture on the scope in the sites where it was in contact with some surface area of the AER, and those surfaces will thus remain damp after the air purge cycle in the AER.
Another frequent observation in the Ofstead study (2010) was the use of linting cloths being used to dry the outside of the scope and accessories such as reusable valves used with the scopes. The flexible endoscope has numerous moving parts, portals to attach buttons and valves, and entry ports for accessories used during the endoscopic procedure that can snag and cause lint to stay attached to the scope. Cloths that produce lint, such as terry towels, can catch on the metal rims of the ports or in the components of reusable buttons and valves; they can also leave fragments of material between the knobs.
These fragments allow moisture to remain and provide an environment for microbial growth. Using a linting cloth presents a risk for threads or lint that have been left behind on the scope to be introduced into the next patient during irrigation or when accessories are pushed down the working channel. The best option for hand drying is a microfiber cloth, since it is very low linting.
Syringe air used to dry the channel is not effective in producing a dry channel. Research has shown that the pressure is just not sufficient to make sure the air moves the residual moisture to the tip of the scope. Remember, all professional society guidelines advocate against the use of syringes for drying.
Only low-pressure instrument air should be used if you are doing manual drying. Pineau et al. (2008) compared the efficacy of two minutes vs. 10 minutes of drying time on subsequent microbial growth, The study demonstrated that with only two minutes of drying, there was microbial growth at 48 hours, whereas ten minutes of drying showed no microbial growth after 72 hours.
Human Error – Storage and Handling
Scopes touching walls, the floor of the cabinet or other scopes are consistently identified by auditors doing accreditation surveys as areas where a Level I recommendation for improvement may be cited.
Many people do not understand how external contamination happens. As mentioned earlier, not wearing clean gloves to move the scope into or out of storage can contaminate the surface of the scope. (Best practice would suggest that a scope should never be handled without wearing some type of glove. SGNA in 2018 explicitly added this guidance to handling of clean scopes.) You cannot determine if a scope has been reprocessed simply by viewing it. Handling a scope without clean gloves not only could transmit microbes from your hands to a scope, which later could be transmitted to a patient, it could put you at risk of handling a contaminated scope and inadvertently transmitting infection to yourself.
Even if you are using clean gloves, there are storage risks inherent in the long, unwieldy construction of flexible endoscopes. If there are staff who are shorter in height, they may have difficulty hanging scopes in a storage cabinet because they can’t access the hangers. (We have seen people use one of their feet to lift the tip of the scope into the cabinet!)
Because space is limited, there may be too many hangers in the cabinet, making it difficult for the person to either enter a scope or remove it without touching other scopes. All of this undesirable handling can contaminate the scope with bacteria from the environment or even from a different scope.
Human Error – Inspection and Maintenance
Fluid and moisture can be retained in the scope if it does not have regular maintenance checks for leaks. Ofstead (2013) found that the most frequently skipped step in processing scopes was leak testing. Failure to discover a leak can allow fluid to infiltrate the working elements of the scope, which will necessitate expensive repair; more significant in this context, a leak allows moisture to collect and support bacterial growth.
Preventive maintenance on endoscopes (as well as other equipment) should be done according to manufacturer IFU schedules. Manufacturers recommend a regular schedule of maintenance on functioning equipment to decrease the chances of it failing during use. Preventive maintenance can replace worn components, clean and lubricate parts, and even find and repair damage that hasn’t yet become symptomatic. It is considered an important strategy for infection prevention. Skipping preventive maintenance to “save money” is a penny-wise, pound-foolish approach; the cost to the facility to repair a major defect or to care for patients who’ve been injured by faulty equipment will far exceed the outlay for regular upkeep of equipment.
There are many critical touch points in endoscope processing. The evolution in research and practice regarding three of those touch points has been discussed here. The latest evidence tells a very different story about the impact of drying and storage than what many of us were taught when we first began working with flexible endoscopes. It is important for all of us who work with flexible endoscopes to remember that each step in the processing cycle builds on the previous step. If drying wasn’t done properly, then subsequent handling and storage can put the scope at risk of microbial growth from environmental organisms.
Finally, it is incumbent on the people working with flexible endoscopes to pay attention to guideline updates on issues related to endoscope processing and to stay up to date on new evidence that comes from peer-reviewed, published studies. The case in point is that cleaning and drying are now considered equally important in creating and maintaining a patient safe scope. We’ve come a long way from hanging a scope on a hook and hoping for the best.
Alcohol purge: 70-90% isopropyl alcohol used to expedite drying in reprocessed flexible endoscopes.
Bioburden: The degree of microbial load; the number of viable organisms contaminating an object.
Biofilm: A thin layer of microorganisms adhering to the surface of a structure, which may be organic or inorganic, together with the polysaccharides that they secrete.
Borescope: A device used to inspect the inside of an instrument through a small opening or lumen of the instrument.
Conventional storage cabinet: Dedicated cabinet designed for the protection and temporary storage of high-level disinfected, dried endoscopes until they are used on a patient.
Critical Water: Water that is extensively treated to remove microorganisms and other materials.
Decontamination: The process of cleaning and decreasing the level of bioburden on a device or instrument.
Disinfection: The process of cleaning something, especially with a chemical, in order to destroy bacteria
Drying cabinet: A validated medical device, designed for active drying and storage of flexible endoscopes, that circulates continuous filtered air through each endoscope channel and within the cabinet, and meets the EN16442 standard for proof of drying within a specified timeframe.
Endotoxin: A toxic substance present in the outer membrane of gram-negative bacteria that is released from the cell when it disintegrates
Endogenous: Microorganisms that reside inside the body
Exogenous: Microorganisms that are in the environment
High concern microorganism: Organisms often associated with disease, such as gram-negative bacteria (e.g., E. coli, K. pneumoniae, Enterobacteriaceae, P. aeruginosa), and gram-positive bacteria (S. aureus, and Enterococcus). Positive cultures of organisms of high concern require corrective action.
High level disinfection: Processes that kill all microbial pathogens, but not necessarily all bacterial spores.
Infection Control Risk Assessment: A documented process to proactively identify and plan safe design elements, including consideration of long-range infection prevention; identify and plan for internal and external building areas and sites that will be affected during construction/ renovation; identify potential risk for transmission of airborne and waterborne biological contaminants during construction and/or renovation and commissioning; and develop infection control risk mitigation recommendations to be considered.
Instrument grade air: A medical gas that falls under the general requirements for medical gases as defined by the NFPA 99: Health Care Facilities Code, is not respired, is compliant with the ANSI/ISA S-7.0.01: Quality Standard for Instrument Air, and is filtered to 0.01 micron, free of liquids and hydrocarbon vapors, and dry to a dew point of -40° F (-40° C).
Log reduction: A 10-fold reduction in the number of live bacteria. A 1-log reduction would reduce 100 bacteria to 10, or 10 billion bacteria to 1 billion.
Low concern microorganism: Organisms less often associated with disease and potentially a result of contamination of cultures during collection, such as coagulase-negative staphylococci. Levels of low-concern organisms can vary depending on the processing procedures in the facility
Microbial growth: How rapidly microorganisms can reproduce.
CNE Certificate Process
Note: Your computer should be connected to a printer before completing the Post-Testing in order to permit printing your course certificate.

