DISCOVERING YOUR SCOPES’ HIDDEN SECRETS
Overview
Guide to Study
eLearning Activity
Processing of reusable medical devices after use can be challenging. That is especially true for flexible endoscopes. Flexible endoscopes have multiple internal channels as well as openings such as ports and movable working elements. Part of determining if a scope has been cleaned adequately and is ready for the next procedure is to verify the cleanliness of the device after cleaning and before high level disinfection or sterilization, which means looking for debris remaining on or in the device. Additionally, you want to look for microscopic damage before the scope is high level disinfected or sterilized.
Visual inspection of any medical device is critical during processing. Inspection plays a pivotal role in determining if a medical device is clean enough and structurally intact enough to undergo high level disinfection or sterilization. It is especially important to inspect internal channels if possible and to inspect external surfaces where debris could possibly reside in dents, scratches, and crevices. Research-based evidence has been published that documents the significant amount of bioburden loading on procedurally used medical devices and the amount of bioburden that is removed during the cleaning of these devices. This program discusses the important role visualization plays in flexible endoscope reprocessing and provides strategies for utilizing available technology to achieve the highest level of success in validating the cleaning process outcome.
There are numerous recently published articles related to visualization and the outcomes of flexible endoscopes after being processed. Each article brings a different perspective of findings and how they could contribute to patient safety. Many findings are consistent throughout the articles.
Ofstead and associates are well known researchers associated with flexible endoscope reprocessing practices. In 2016, Ofstead et al. were able to determine the cleaning efficacy in gastroscopes and colonoscopes. They found gastroscopes were more contaminated than colonoscopes. Another finding was the most contaminated portions of the scope were biopsy and suction ports. Furthermore, using a borescope, they were able to determine if there was damage and/or residual debris. Another study completed by Ofstead and associates used adenosine triphosphate (ATP) to verify determine cleaning efficacy followed by visually inspecting the channels after cleaning. Their findings showed multiple fluid drops in the scope as well as in and around the ports tested. The borescope showed what seemed to be simethicone residue. The ATP results showed colony forming units were low and nonpathogenic or non-disease producing.
Ofstead et al. (2017) also studied guideline compliance during flexible endoscope processing. On initial visualization using a borescope, they found 17/20 scopes needed to be sent for repair. They also found fluid in 19/20 scopes. The authors suggested all scopes sustain damage as they are used and there needs to be a better understanding of what level of damage can be sustained before a scope should be sent for repair. Using lighted magnification for externally inspection of the scope’s working surfaces and a borescope for viewing the internal channels will help the staff member be better equipped to determine the usability of a scope.
It is not only gastrointestinal scopes that have been studied using visualization. Ofstead et al. looked at 16 ureteroscopes from two institutions. Visualization inspections identified debris protruding from channels, oil, deposits, white lint, and foamy residue.
During 2019 Ofstead et al. looked at the effectiveness of endoscope reprocessing across four facilities located on the East and West coasts and in two Midwestern states. They found fourteen out of twenty-four bronchoscopes (58%) had microbial growth after reprocessing. There was residual fluid in twenty-two out of forty-nine endoscopes (49%) while 100% of the scopes had defects. Oily residues and tissue glue from a procedure were also found.
Thacker et al. did a pilot study using the SteriCam (borescope) and the use of additional forced air drying. During the visualization process, they used a borescope and were able to determine fifty-one out of fifty-nine (86%) had scratches and thirty-five out of fifty-nine (59%) had shredding and discoloration. They also found twenty-two out of ninety-seven (23%) had debris in the channels. While 35% (eight out of twenty-three) of the scopes (non-elevator) had visible moisture, after 2 minutes of forced air drying per port there was 0% moisture.
Visrodia did a review of articles for observations on visualization and inspections of flexible endoscopes. His reviews determined instrument channel findings appear to be common and include scratches, discoloration and residual channel fluid. He also suggested that residual moisture has been shown to promote microbial growth. These were common findings throughout the published research. He suggested the borescope may provide an effective method for determining dryness of flexible endoscopes.
Barakat et al. looked specifically at the impact of drying on flexible endoscopes. They found significantly more droplets were evident after manual drying for five and ten minutes compared to automated drying. Manual drying included the wiping down of external surfaces before storage in a traditional (vertical hanging, no air flow) cabinet. Automated drying involved using a low pressure HEPA filtered air device. ATP levels were higher at 48 and 72 hours for manual drying compared to automated drying. Automated drying had fewer droplets after 5 minutes of drying using the automated system while there was minimal fluid visualization at 10 minutes of automated forced air drying.
All these studies reflect the importance of visualization of the flexible endoscope after cleaning and before it is high level disinfected or sterilized. At a minimum, learning to use lighted magnified inspection should be considered a critical step to verify the cleaning process; additional implementation of borescope inspection would be an even stronger commitment to verification of cleaning outcomes.
All professional society guidelines recommend visualization of the flexible endoscope after cleaning and before the scope is high level disinfected or sterilized. The Society of Gastroenterology Nurses and Associates (SGNA) specifically calls out visualization as a “safety time out” before the scope moves on the next step in reprocessing. Doing the visualization can accomplish a couple of things. First, it prevents the scope from having an inadequate surface preparation for high level disinfection or sterilization. Second, it prevents wasting money on an additional high-level disinfection cycle that needed to be run because the damage or debris was discovered when the scope was removed from the automated endoscope reprocessor. The recommendations from three professional society organizations are listed in the following table.
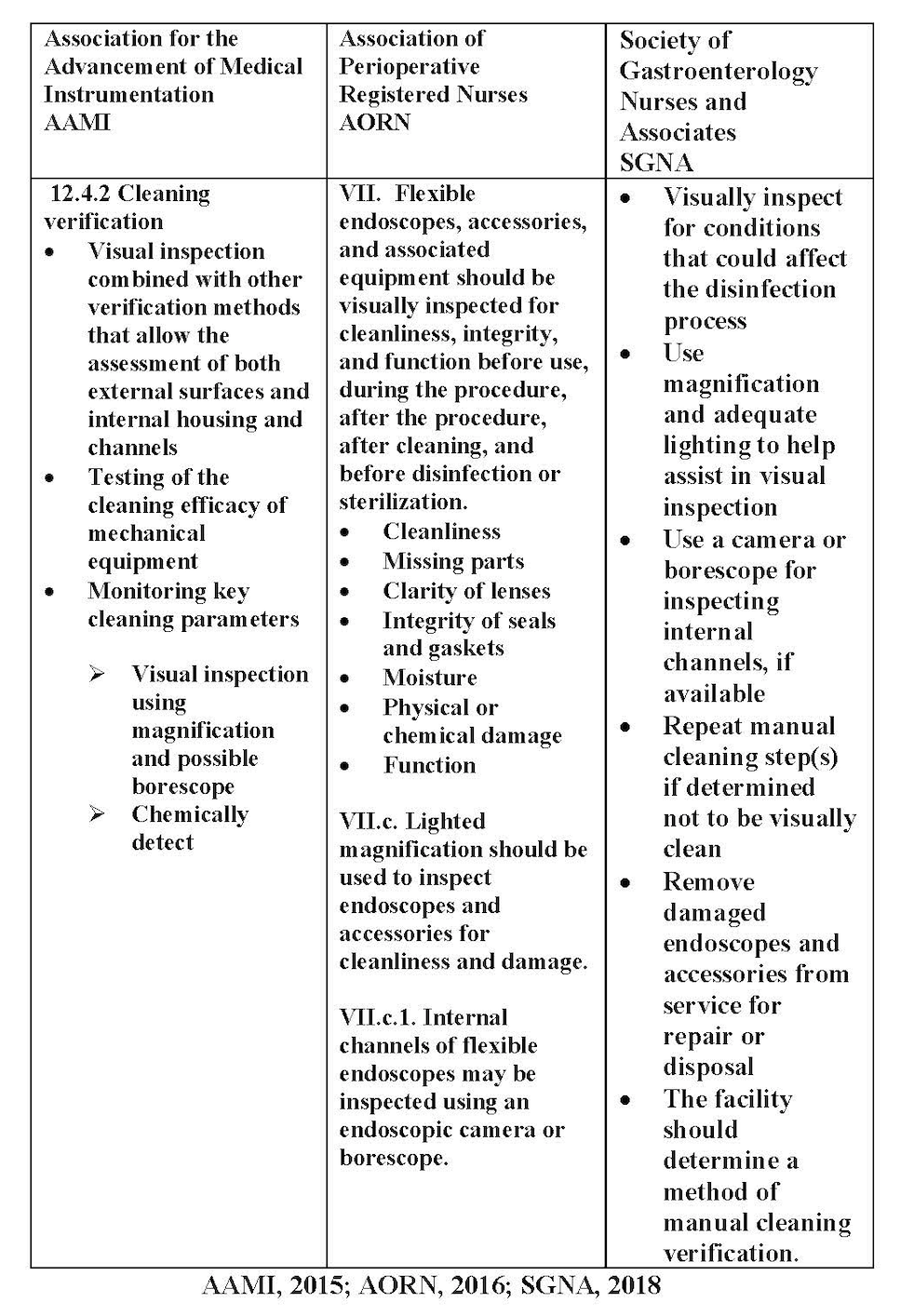
Manufacturer’s Instructions for Use (IFU) suggest every time a scope is handled the staff member should be looking at the scope for defects, tears in the sheathing and damage. Professional society guidelines such as AORN and SGNA specifically call for lighted magnification at a minimum and additionally to use a borescope when possible. As noted above, SGNA considers visualization as a “safety time out” during processing of a flexible endoscope.
All scopes are at risk for damage each time they are used or handled. Many healthcare facilities currently use lighted magnification for visualization. However, in some of those facilities, colonoscopes, gastroscopes, and bronchoscopes may not receive the same treatment as the more advanced scopes. Many facilities choose only to perform magnified inspection of the duodenoscope or the advanced ultrasound scopes, both of which have elevator channels. Every time a scope is used there is the potential for damage to occur, irrespective of whether it has an elevator channel or is a more straightforward design.
In any process, checklists have been acknowledged as one of the best ways to make sure all critical steps have been completed; in fact, they have been used in the airline industry for decades. The healthcare industry utilizes checklists in surgery to make sure all critical care points have been covered. These checklists are especially important for surgical patients who are anesthetized during their procedures and have no voice in order to make sure everyone understands what is happening with this patient and what the patient has consented to have done. The team must be in agreement on the care and procedure process before the surgical procedure begins. Reviewing the checklist by the team makes sure everyone has a clear understanding of what is to be done.
In the same vein, using a checklist in flexible endoscopy provides a visual reminder for each part of the scope that needs to be inspected. Referring to a checklist is a quick and easy way to make sure all parts of the scope have been inspected.
Staff members tasked with the processing of flexible endoscopes have the same responsibility for completing each step involved in processing for every scope. Frequently, processing staff may be interrupted or called away to do something else while in the middle of processing a scope. They may or may not remember where they stopped; if that happens, they may have to start all over again. Visualization of all the parts of the scope is a final verification of cleaning before the scope is placed in the high-level disinfectant or packaged for sterilization. If there is any remaining debris or if damage has been identified, the scope can be recleaned or pulled from the inventory for repair before completing the process. Note that even though a damaged scope must be processed before being sent out for repair, the staff member is aware of the damage and can handle the cleaning and disinfection appropriately as recommended by the scope manufacturer’s instructions for use on returning a damaged scope.
External device target inspection
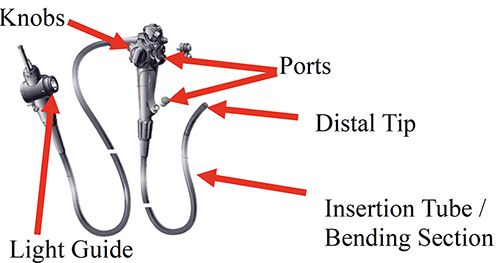
There are multiple imaging devices on the market to help the staff member better visualize both the external and internal components of the scope. The Association of periOperative Registered Nurses (AORN) recommends that during inspection the scope should be visually inspected for cleanliness, missing part, clarity of lenses, integrity of seals and gaskets, moisture, physical or chemical damage, and function. The parts of the scope that need to be inspected externally include the button and valve ports, moving knobs, light guide plug, bending section, and the distal tip. Buttons and valves are constantly being inserted and removed during a procedure and in procedure setup. This activity can cause scratches or damage that makes the ports unsafe or provide a harbor for microorganisms to grow. The moving knobs can hide bits of debris or strings from the gauze sponges used during a procedure. The light guide plug should always be inspected to make sure no prongs are missing and there is no other visible damage. A fluid resistant cap should be attached if applicable after inspection and before placing the scope in the high-level disinfectant. The bending section is where damage can occur particularly easily; this section of the scope receives a great amount of abuse during a procedure as it is twisted and turned in all directions in order to drive the scope forward and backward during a procedure. Finally, the distal tip can be damaged from instruments being inserted and withdrawn through the working channels. For example, the spray nozzle can be damaged and could become very sharp, contributing to a future patient injury such a laceration of the gastrointestinal tract.
Even though these are the most likely places for damage, the processing staff member should also look at the umbilicus and the insertion tube to identify any wear and tear of the scope. Early detection can prevent putting a patient at risk for microbial exposure or transfer and will eliminate wasting disinfectant and time related to a second reprocessing of the scope. On top of those benefits, if damage or a leak is discovered early it is much cheaper to fix than later when it could require a major repair.
External Device Target Inspection
There are multiple types of lighted magnification devices. Hands free devices allow the staff member to handle the scope and manipulate different parts during the examination. Central Service Departments have used these hands-free lighted magnifiers for a long time. Other options for lighted magnifications include headset glasses and handheld magnifiers. All increase the level of visualization. Guidelines recommend a 10x magnification for best accuracy.

Internal visualization devices
Internal visualization can present the biggest challenge. A borescope is one device that can be used for internal visualization of the scope channels. There are multiple types of borescopes. The type used in flexible endoscopy is a long flexible scope without a lumen that is passed through the scope’s channels to allow the staff person to identify damage such as scratches, tears, dented tubing, debris, or droplets such as simethicone left in the channels after cleaning has been completed. The borescope essentially allows you to scope the scope. Some borescopes are short and must be inserted at the air/water and suction ports and then removed and inserted into the distal tip. A long borescope should be able to be inserted for the entire length of the channel, needing only one pass to inspect for damage, fluid, and debris. Some borescopes use analog data to view the scope channels while others use digital technology to enhance visualization.
Digital viewing gives a much clearer picture compared to analog views. Multiple manufacturers sell the borescope products. Borescopes have a variety of viewing modalities - some include an integrated video screen while others utilize a computer screen to monitor the imaging. A borescope’s ability to record and track images is another factor that should be considered when comparing the different types of borescopes on the market. Make sure you know the diameters of your scope channels and the size of the borescope before you insert a borescope into an endoscope channel!
Borescopes

When you purchase a borescope make sure your staff receive adequate training to determine what they should be looking for and what level of damage would be significant enough to send a scope for repair. The manufacturer representative should be able to educate the staff about using an internal device by making sure they know what to look for and what to do about it. Ofstead (2017) suggested there needs to be additional studies to determine criteria for determining when a scope must be sent for repair. Completing a leak test properly is an additional check and balance for making sure the scope is in working order.

In the examples of visualization above, you can see different types of damage that have occurred to a flexible endoscope, including a dented tube and slices in the sheathing. There are examples of discoloration which could be rust or blood. Debris left behind in the channels indicates that the brushing and flushing phase of cleaning was incomplete or unsuccessful. The debris could be clips, tissue, or pieces of brushes. One of the most important opportunities for visualization identification is to determine if the scope is dry. When scopes have not been exposed to automated delivery of pressurized air, there may still be fluid drops left in the scope’s channels. If simethicone was used during a procedure, residual simethicone droplets may also be found.
It is important to view the process of visual inspection with a borescope as surveillance rather than a single inspection. Over time, thorough surveillance, the technician will notice changes to the inner lumens of the device. True, the first time a borescope is used in a particular endoscope there may be significant findings. After the initial inspection and documentation of findings, the technician’s role is to surveil the scope to monitor for small changes and any abnormalities from regular use.
Another important aspect of proper visualization is the lighting within the reprocessing environment. As we grow older our eyes do not have the same acuity as when we were younger. The age of the staff member, the colors used in the design of the work environment and the speed and accuracy of the work required play an important part in determining the amount of illuminance needed in the work area. Illuminance requirements can change as the tasks change. Most tasks in the reprocessing areas require detailed and accurate inspection. The Association for the Advancement of Medical Instrumentation has provided some guidance on recommended lighting levels, which can be seen in the following chart.
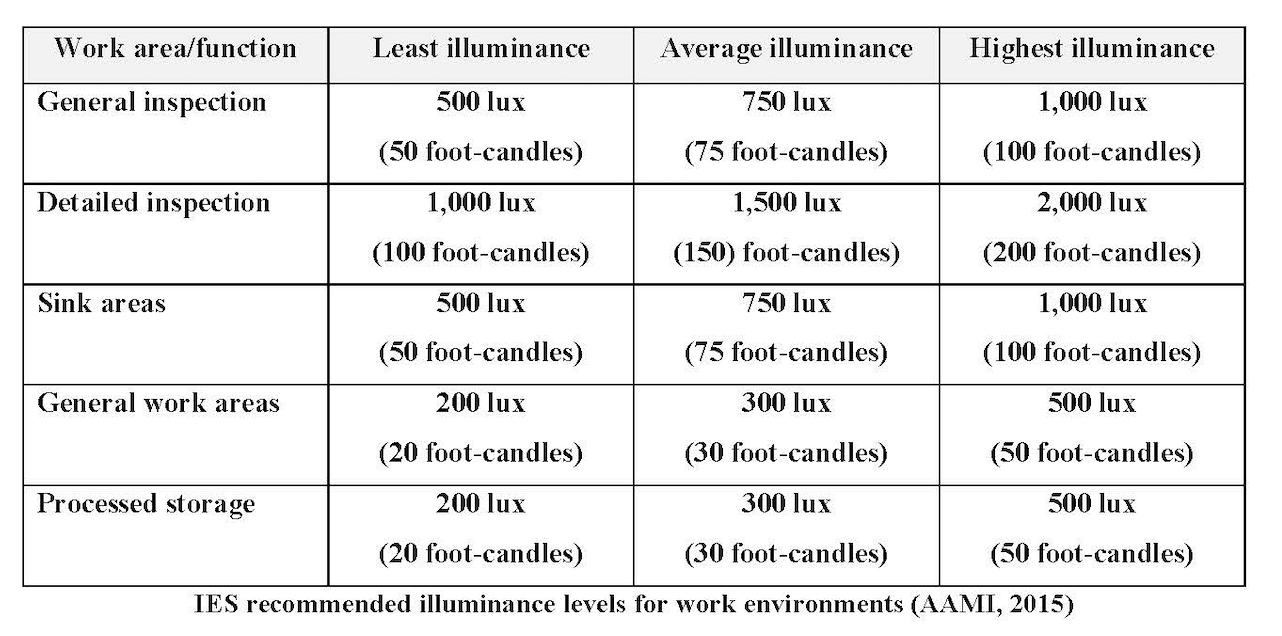
As we grow older our eyes do not perceive as sharp an image as when we are younger. All guidelines recommend lighting to be appropriate to the level of work being completed and for the minimization of eye strain for the worker. Using lighted magnification allows the staff member to identify damage to the scope much faster and more easily.
Visual inspection is not the only way to measure for cleanliness, surface damage, and residual fluid. There are other tests available to verify cleanliness such as a protein, hemoglobin, or ATP. These tests only measure residual types of debris or enzymes. The cobalt blue litmus test will measure for moisture inside the channels or if there is water present on external surfaces. However, these tests do not replace visual inspection, but instead work as an adjunct method for verification and process improvement.
Currently there is no requirement for documentation of visualization of the flexible endoscopes. However, education and training documentation should reflect the staff member has been given training in this area. Policies and procedures should include visualization as part of the complete flexible endoscope cleaning process. Staff members who have received certification either from the International Association of Central Service and Materials Management (IAHCSMM) or the Certification Board for Sterile Processing Distribution (CBSPD) have been educated to the cleaning verification through visualization process.
Visualization has been identified by all professional society guidelines as a critical part of making sure flexible endoscopes have been adequately cleaned, with no residual debris in channels or on external surfaces and are free of damage. The visual inspection is done after cleaning and before the flexible endoscope is sent for high level disinfection or sterilization. Education and training are necessary to help the staff member determine how to use visualization devices. Identifying scopes that have not been adequately cleaned or have sustained damage can prevent a future patient from experiencing a bacterial exposure or transmission.
Bioburden: The degree of microbial load; the number of viable organisms contaminating an object.
Borescope: A device used to inspect the inside of an instrument through a small opening or lumen of the instrument.
Disinfection: The process of cleaning something, especially with a chemical, in order to destroy bacteria.
High level disinfection: Processes that kill all microbial pathogens, but not necessarily all bacterial spores.
Instrument grade air: A medical gas that falls under the general requirements for medical gases as defined by the NFPA 99: Health Care Facilities Code, is not respired, is compliant with the ANSI/ISA S-7.0.01: Quality Standard for Instrument Air, and is filtered to 0.01 micron, free of liquids and hydrocarbon vapors, and dry to a dew point of -40° F (-40° C).
Microbial growth: How rapidly microorganisms can reproduce.
CNE Certificate Process
Note: Your computer should be connected to a printer before completing the Post-Testing in order to permit printing your course certificate.

