Know Your Cleaning Chemistries
Overview
Guide to Study
eLearning Activity
Historically, guidelines and recommendations were developed and implemented on experience and common sense. As research has become more prevalent, the use of evidence-based research has increased and provides a scientific knowledge base for decision making. Researchers now provide comparisons of chemistries, identifying strengths and weaknesses of chemistries used in flexible endoscope cleaning and reprocessing. The focus for this CNE presentation is on the chemistries used for cleaning flexible endoscopes before they are sterilized or high level disinfected. The discussion will involve the different types of water and detergents used for cleaning flexible endoscopes. Each subject brings a unique set of considerations for determining your choice when you are responsible for flexible endoscope cleaning.
There are five recommended steps using water and detergents before the scope is ready for high level disinfection:
- Pretreatment at point of use (formerly called precleaning)
- Contaminated transport
- Leak testing
- Manual or automated cleaning
- Rinsing
Each of these steps will be discussed individually in the following section.
The human body is made up of approximately 39 trillion bacterial cells and 30 trillion human cells (AAMI, 2016). Some of the microorganisms are good and some are bad. Flexible endoscopes become contaminated during endoscopic procedures. The bioburden loading can range from 1log/cm2 to 10 logs/cm2 (Alfa 1991, Ren 2013). Removal of this bioburden is critical to preparing the internal and external surfaces of the scope for high level disinfection and storage before being used on the next patient.
Importance of Pretreatment
Evidence has shown that if pretreatment is not done at point of use, biofilm formation can begin and debris can dry on the scope, making it more difficult for the reprocessing staff member to remove bioburden. Additionally, all professional society guidelines, i.e. the Association for the Advancement of Medical Instrumentation (AAMI), the Association of periOperative Registered Nurses (AORN), the Society for Gastrointestinal Nurses and Associates (SGNA), and the Multi Society Guidelines recommend pretreatment at point of use.
Pretreatment consists of alternating air and fluid through the channels as soon as possible once the scope is removed from the patient. Each scope manufacturer provides specific details through Manufacturer’s Instructions for Use on what they consider appropriate pretreatment for their flexible endoscopes after they have been used. Usually this will involve two steps, wiping the scope with a sponge, low linting wipe or low linting cloth from the cleanest to dirtiest part of the scope and then suctioning a liquid such as water or a detergent solution, followed by air, followed by solution again. Alternating these two methods generates agitation within the channel and aids in the removal of the debris. Completing the step in a timely manner helps remove the debris and aids in keeping the channels moist (but not wet) during contaminated transport and while waiting for the cleaning process to begin. This is the first of nine identified steps in flexible endoscope reprocessing recommended by guidelines and manufacturers.
Contaminated Transport
The initial point of use pretreatment removes gross debris from the scope and allows the scope to maintain moisture (but not water) in the channels. This ensures that residual contaminants do not dry on the surface of the scope or in the channels. We need to provide protected transport of medically contaminated medical devices.
Contaminated endoscopes should be transported in a container that protects personnel and the environment from droplet and airborne particles. If a plastic bag is used for transport, care should be taken in scope placement, as gravity of the heavy components and loops increase potential for bag puncture and compression damage to the scope. Covered hard plastic containers should be large enough to allow loose coiling of the scope, without placing stress on the tubes. The Occupational Safety and Health Administration (OSHA) has mandated that a Universal Biohazard Symbol be visible when contaminated medical devices are transported. Transporting in a rigid container with a barrier covering the medical device protects the transporter as well as the medical device.
Consideration should also be given to how the scope is protected once it reaches the decontamination area. Avoid stacking bagged scopes awaiting reprocessing to prevent damage.
Leak Testing
The third important step is leak testing. Leak testing should be done before the scope is manually cleaned or before automated cleaning occurs. Leak testing verifies the scope has not sustained any damage to the integrity of the scope channel lining or the external sheathing surface of the scope. It can be completed using a manual dry or wet leak test method or an automated dry or wet leak test method.
A dry manual test uses a pressure gauge similar to the one attached to a blood pressure cuff; pressure should be maintained even when the bending tip is moved in all directions.
For the wet manual test, the pressure device is attached, and the scope is inflated and then the scope is submerged in clear water (i.e., water with no detergent). A low lint cloth should be used to remove all bubbles from the surface of the scope and fluid should be flushed through the channels till it is visibly flowing from the insertion tip.
After pressurizing the scope, you should look for any bubbles while the scope is at rest and then during each angulation or bending, allowing time for bubbles to appear at each bend. Automated dry leak testing allows for consistency in determining leaks and provides a documented outcome. Automated wet leak testing also allows for an increased level of consistency but may not have a printout to verify passing the leak test.
Leak testing is critical to prevent fluid invasion and to prevent debris and organisms getting into the working elements of the scope. If microorganisms are trapped in a hole or tear in the scope, they cannot be removed during cleaning and will not be exposed to the high-level disinfectant. That reservoir of microorganisms has the potential to infect subsequent patients. Discovering a leak early prevents the potential for an exposure or transmission to a patient and decreases the cost of a repair.
Manual or Automated Cleaning
Cleaning is a critical step in preparing the surface and internal channels of the scope to come in contact with the high-level disinfectant. Cleaning provides a significant reduction in bioburden, making it easier for the high-level disinfectant to kill all remaining bacteria, fungi and viruses so the scope will be safe for use on the next patient.
When cleaning flexible endoscopes after you have completed the leak test you have two options: manual cleaning or automated cleaning. The main challenges for manual cleaning are selecting the appropriate detergent that is approved for medical device cleaning, using clean detergent solution at the correct concentration, and remembering that detergents have a specific contact time, according to temperature. When you are doing manual cleaning, you should read the manufacturer’s Instructions for Use (IFUs) to determine the concentration necessary to be effective, which temperature is most effective, and how long the endoscope must be in contact with the detergent, both the internal channels and the external surfaces. Following the IFU helps makes it easier to do your job correctly, and also meets recommended guidelines for following IFUs for detergents.
Use a low lint cloth to clean the external sheath of the scope and use cleaning devices specifically designed for cleaning the ports and turning knobs. Use brushes, Pull Thru™, or other accessories to clean the scope channels. An automated flushing device can replace syringes for the flushing and rinsing step of the manual cleaning.
Automated cleaning is done in an automated endoscope reprocessor (AER). Not all AERs have been cleared by the Food and Drug Administration (FDA) to clean scopes. Some AERs may be able to complete some steps in cleaning. READ the Instructions for Use from the AER manufacturer to determine the cleaning capabilities of your AER. And again, be sure you follow IFUs to verify that you have the correct concentration of the correct detergent solution, at the correct temperature and for the appropriate contact time to thoroughly clean the scope.
Detergent Requirements for Flexible Endoscope Cleaning
The responsibility of cleaning flexible endoscopes is challenging to say the least. It's very important to understand how the scope becomes contaminated and what type of contamination is present. When you know the type of soil that is present, you can decide what type of detergent to use in the cleaning process. Just like at home where you use a specific type of soap for dishes, dishwasher, or laundry, so too should you know about detergents for medical device reprocessing. Most medical device manufacturers will recommend a low foaming, pH neutral, or slightly alkaline detergent that may include enzymes and/or surfactants. It should be able to rapidly penetrate the type of soil that is present. Quality of water used will also have an impact on the efficacy of the detergent.
Rinsing
After cleaning there needs to be a thorough rinse. This rinse can be done with regular tap water. Make sure all surfaces, valve ports, turning knobs, and channels are rinsed completely. You want to make sure there is no detergent solution left on or in the scope before it is put into the high-level disinfectant. If the scope is going to be manually high level disinfected (HLD) you should dry the scope and purge air through the channels, so it does not dilute the reusable HLD chemistry. If you are using an automated HLD process you may not need to dry the scope first. READ the IFU for the AER.
Documentation Required For Pretreatment and Cleaning
Currently there are no recommendations for documentation of pretreatment at point of use. However, all scope manufacturers recommend a specific cleaning process when there is a delay in the start of cleaning, which may require up to several hours presoaking before cleaning occurs (Olympus, 2019). The staff responsible may have no clear idea of when point of use pretreatment occurred, so unless that information is shared with them when they receive the scope, they may mistakenly assume that pretreatment was performed within the last few minutes and neglect to initiate the delayed cleaning process. One simple way to communicate is to write the time and name of the person doing pretreatment on a patient sticker, and routinely send with the scope so the patient information can be entered into a log book (for manual cleaning) or the automated endoscope reprocessor (for automated cleaning). The staff now have enough information to make an informed decision on which protocol to follow in the cleaning process.
Now that you have the steps required for pretreatment and cleaning let’s review the properties of chemistries.
Water
Water is one of the most used chemistries (H20) in medical device cleaning, but its quality variations are not always clearly understood. There are various types of water used in the cleaning process.
The most frequently used category of water is tap water. Tap water comes from the faucet. It may have been treated through filtration upstream or where the water enters the facility or department; alternatively, it may have had chlorine added to the local regulatory specification to inhibit bacterial growth. If the water has been filtered, the filter typically used for tap water is a particulate filter which catches large mineral or debris in the water line system. It may be called a 0.45-micron filter. Particulate filters do little to filter out microorganisms. Tap water is also referred to as potable water, meaning it is drinkable. There can be microorganisms in the water measuring up to 500 colony forming units/cm2 (CFU/cm2).
Tap water is one of the most common sources of water for everyday use. Unless you live in a rural area, it is usually controlled by public utilities. Tap water is most often used for cleaning medical devices and then for rinsing them before they are sent for the next step in reprocessing.
Reverse osmosis water is another type of water used in medical device reprocessing. It is water that has been filtered through a membrane or multiple levels of filters, allowing almost all the minerals deposits, particulate matter, organic molecules and pyrogens to be removed. RO water is recommended for final rinses if possible. It is the best type of water for the whole flexible endoscope cleaning process.
Deionized water is created through a chemical process that uses ion-exchange to eliminate dissolved mineral. Deionizing does not remove all contaminants and does not filter out bacteria. It is expensive to run and maintain.
Sterile water is created by turning filtered water into steam and then collecting the condensate. This water would be entirely free of microorganisms and particulates. Sterile water is generally used for irrigation and meets a USP standard as a solvent for injectable drugs. In the reprocessing of medical devices, sterile water OR critical water (water that has been extensively treated to remove particulate and microbial inclusions) would be used to rinse high-level disinfected or sterilized scopes and accessories.
Water quality plays an important role in cleaning as well as rinsing. Some factors you should consider when you are using water: the water hardness, pH level, water temperature, ionic contaminants, microbial level and bacterial endotoxins. "Hard" water can leave mineral deposits on freshly cleaned medical device surfaces. Desirable pH levels for water should be in the neutral pH range of 6 to 8. Water temperature is more important for detergent use; enzymatic detergents may require a warmer temperature than non-enzymatic detergents. Ionic contaminants such as chloride or heavy metals can cause corrosion of the instruments and should be avoided. High levels of microbes and bacterial endotoxins can be present in water that is not filtered. High purity water should be used as a final rinse for high level disinfected medical devices. Most automated endoscope reprocessors have a filter process with a particulate filter of 0.45 microns (0.45µ) and a bacterial filter of .02 microns (0.2µ). Answers for key questions about water can be found in the AAMI TIR34:2007.
Introduction to Detergents
Cleaning is considered the most important step in flexible endoscope reprocessing. There are two specific steps where detergents are used for cleaning. The first is at point of use or pretreatment. At this step, the scope is wiped externally with a moist low linting cloth with or without detergent depending on the scope manufacturer’s Instructions for Use at the point of use pretreatment. A cleaning solution (i.e., water and/or detergent solution) is then alternated with air to cause agitation and to assist in removing bioburden from the internal channels of the flexible endoscope. The scope must be connected to the scope processor in order to use of the suction and air. The second area where detergents are used is in the cleaning process step. There are many types of detergents available for use on flexible endoscopes. For this program we will discuss three most frequently used detergent categories: enzymatic, non-enzymatic surfactant and alkaline plus surfactant.
All detergents assist in wetting of and penetration into soil and in containment of the removed material in a suspension. A detergent is a cleaning agent that loosens debris from surfaces, holds debris in suspension so it doesn’t redeposit onto the surfaces and allows the debris to be easily rinsed away. The ideal properties of a medical grade detergent include low foaming, rapidly penetrates and solubilizes blood, proteins, cellular mucosal debris, carbohydrates and lipids, removes debris from a variety of surface types, and has a neutral or slightly alkaline pH. They should also be non-allergenic and biodegradable. Detergents will often contain buffers and chelating agents which are used to provide compatibility with materials, reduce effects of hard water and prevent mineral deposits from remaining on the instrument and finally, to allow water to run off in sheets, leaving no droplets or spots. A comparison example of this action would be using Rain-X® on your car’s windshield. When you use this product, rain tends to move across the windshield in sheets rather than big splashes, allowing you a clearer view of the road during a storm.
Each type of detergent has a specific way it works on bioburden. Enzymatic detergents have been used since the early 1950s to remove stains from clothing. In medical device detergents, enzymes are used to remove specific types of tissue.
Most enzymatic detergents have protease, amylase and lipase enzymes. Protease enzymes are attracted to protein substances such as blood, mucus and urine, while amylase enzymes work on carbohydrates. Lipase enzymes work on fats. All these types of tissue may be seen during different endoscopy procedures. Enzymatic detergents work best at warmer - not hot - temperatures and may require a slightly longer contact time to be effective against bioburden removal. Doing point-of-use pretreatment immediately after the completion of a procedure is important not only to remove gross bioburden but also to keep the channels moist (but not wet) during contaminated transport, and while waiting to begin the cleaning process in the decontamination room.
All detergents require specific contact times, temperatures, and concentration. Frequently, the manufacturer will suggest a range of temperatures and this range could impact the contact time. Usually cooler temperatures require longer contact time to be the most effective. Concentration can also impact the effectiveness and efficiency of the detergent. Water is a critical component of the detergent to help soften residual bioburden so detergent can break up the bioburden.
 Enzymatic detergents work on bioburden by breaking it up and penetrating from the top down. Do you remember the classic video game called PACMAN™? In it, the little yellow Pac-Man chases ghosts; when a ghost is caught, Pac-Man devours it. Enzymes work in a similar fashion.
Enzymatic detergents work on bioburden by breaking it up and penetrating from the top down. Do you remember the classic video game called PACMAN™? In it, the little yellow Pac-Man chases ghosts; when a ghost is caught, Pac-Man devours it. Enzymes work in a similar fashion.
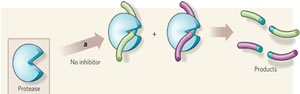
Enzymes break up bioburden and make it easier to be wiped or flushed away. Think of your Sunday brunch and the egg yolk (protein) drying on your plate while you spend time with your family. To clean it, you need enzymatic dishwashing detergent. When you go to the sink, the first thing you do is to let the dishes soak in enzymatic detergent just a bit before you put them into the dishwasher. Sometimes longer contact time with the detergent and warmer temperatures will speed up the process. But too much heat can coagulate the protein. What's true in your kitchen is also true in processing endoscopes.
Non-enzymatic Surfactant based detergents are also known as biofilm detaching agents. They remove soils from the surface by decreasing surface tension and disassociate cells from a surface without breaking them up. They have a neutral pH and contain surfactants (“surface-active agents”), which have both water-loving (hydrophilic) groups and water-avoiding (hydrophobic) groups. This allows them to bind to hydrophobic soils (like oils, parts of proteins, parts of carbohydrates) and make the soils more soluble in water. They penetrate through debris to break bonds between soil and the endoscope wall. They are effective on all substances and effective on contact. They are not temperature dependent as they have a wide temperature range in which they maintain their effectiveness.
The following graphic shows you three stages of a simulated endoscope surface with soil covered by biofilm attached to that surface. In the first picture, the non-enzymatic surfactant-based detergent is hovering in the water; when it notices the biofilm mass, it floats on over and attaches to the tissue and biofilm that you see in the middle. On the right, the non-enzymatic surfactant-based detergent has caused that surface bond to break apart, almost like you put a spatula between the surface and the bioburden. It lifts it off and removes it. The best news? Non-enzymatic, surfactant-based detergents work at room temperature with a one-minute contact time.

Alkaline + surfactant detergents are good at removing organic soils and decreasing surface tension. They may have a high pH and can damage a scope if exposed for long period of time unless buffered accordingly. Alkaline + surfactant detergents are only for use in AERs and are commonly found in Europe.
It is important to know the properties and the characteristics of your detergents. It is always important to read the Instructions for Use from the medical device manufacturer and the chemistry manufacturer. Sometimes name brands are recommended but if the properties of the detergents are the same, either detergent can be used for the medical device. The detergent IFU may also have recommendations for specific medical devices.
Cleaning is not just about chemistries. You need the right tools to be most effective and efficient. The following considerations can improve your cleaning or make your life easier when cleaning flexible endoscopes.
Brushes
The most common accessory should be an assortment of brushes in both size and length. When you think about how a flexible scope needs to be cleaned, you know there are numerous openings as well as varying lengths of channels. The diameter of the channel can be as small as 1.0 mm and may be as large as 6.0 mm. A brush or cleaning device must be able to provide contact with the internal surfaces of the channels. The same is true with the awkward angles where the buttons and valves are placed in the scope. Another consideration is the size of reusable buttons and valves. Their cleaning and reprocessing requirements are just as critical as the flexible endoscope. Always use the Instructions for Use from the scope manufacturer to make sure your cleaning technique is correct.
Flushing Devices
Just as automated leak testers can make your life easier, using an automated flushing device can facilitate cleaning while decreasing the amount of “elbow grease” you need. Each scope manufacturer recommends a set amount of cleaning and rinsing fluid to be flushed or aspirated through each channel. Automated flushing systems replace the need for using syringes of various sizes to push these fluids through the channels. Best practice would be to review these manufacturer Instructions for Use on detergent volume and make sure you use at least as much fluid as the IFU recommends.
A good flushing device will have a quality check to make sure the volume of fluid required by the endoscope manufacturer is actually flowing through the flexible endoscope. Using these automated flushing devices will save wear and tear on your hands and may decrease or eliminate repetitive hand injuries. Syringes may still be required to flush specific sites such as between the knobs or around the ports for buttons and valves.
Low Linting Cloths
A fresh cleaning cloth is recommended for the external cleaning of the scope surface. Lint free cloths are often called for, but they may be hard to come by so low linting cloths are also acceptable. Cleaning with terry cloth is not acceptable. When terry cloths are used, they can shed lint that becomes caught in the turning knobs and the button and valve ports. Those pieces of lint can harbor microorganisms and may become dislodged into a patient during a future procedure.
Dosing Devices
Historically we have used the pump method to add detergent to the water bath for flexible endoscopes. To be more accurate in achieving the correct concentration, best practice would be to use a dosing device. Dosing systems are available from several manufacturers. If you do not have a dosing system, you should use a measuring device to ensure you have the recommended volume of detergent to the recommended quantity of water. Also ensure the water temperature meets the detergent manufacturer’s recommendation.
Low Pressure Instrument Air
Research studies now suggest scopes that are stored vertically to "drip dry" are not as dry as previously thought. Additional drying of all the channels of your scopes may be required before storage. Drying of the channels should be accomplished by using low pressure instrument grade air. A syringe is not acceptable for post-HLD drying because there is not enough pressure or volume to help move the remaining water out of the channels. Using pressurized air delivered through an unregulated nozzle or using anything other than instrument grade air can introduce impurities into the channels and potentially damage the scope channels.
Verification
One important aspect of reprocessing is to verify the results of cleaning and to check for previously undetected damage. This step is done at the completion of cleaning and before high level disinfection starts. If you use an automated endoscope reprocessor with cleaning clearance the cleaning verification cannot be performed as stated in the IFU. However, those machines had to validate cleaning to be cleared by the FDA before sale. An alternative approach is to write into your Policy & Procedure to include careful inspection prior to placement in the AER, before the patient procedure, and following the patient procedure, at point of use.
We will not discuss any of these methods in depth but want to make you aware of several common options.
Option 1: Use magnification to inspect different parts of the scope after cleaning to determine if the bioburden has been removed. Visualization should include the insertion tip, bending portion of the scope, ports and valves, the turning knobs, and the light guide connector. Any of these places can harbor debris and protect residual debris from high level disinfectant contact. Magnified visualization may also identify damage unable to be seen by the naked eye.
Option 2: Use a biochemical test that looks for residual protein, e.g., CHANNELCHECK™.
Option 3: Use Adenotriphosphate (ATP) testing to detect residual debris on the surface or in channels of scopes after cleaning. ATP testing is still a somewhat controversial process as it has not been fully explored for outcomes related to the impact of different detergent types on the readout results.
Option 4: Use a hemoglobin test to measure residual blood on the surface or in the channels.
There are pros and cons for using each of these methods. You need to do your research before you decide on just one method. Ofstead suggests using two methods such as ATP and a protein check to make sure the instrument is clean.
Cleaning is a process and a challenging procedure. It requires completion of many steps, with meticulous attention to detail. Following the correct step sequence and correctly using chemistries and accessories can help you prepare the flexible endoscope to be high level disinfected or sterilized. It is our responsibility as health care providers to keep all our patients safe by being efficient and effective in our job responsibilities.
Bioburden: Population of viable microorganisms on a product and/or a sterile barrier system. When measured, bioburden is expressed as the total count of bacterial and fungal colony-forming units (CFUs) per single item.
Cleaning: Removal of contamination from an item to the extent necessary for further processing or for the intended use. A process that uses friction, detergent, and water to remove organic debris; the process which any type of soil, including organic debris, is removed to the extent necessary for further processing or for the intended use. Cleaning removes rather than kills microorganisms.
Critical water: Water that is extensively treated to remove microorganisms and other materials.
Decontamination: The process of removing pathogenic microorganisms from objects so they are safe to handle, use, or discard (CDC, 2008).
Deionized water: Water treatment process that uses specially manufactured ion-exchange resin to remove ionized salts from the water.
Delayed cleaning: Flexible endoscope manufacturer’s recommended time frame between point of use pretreatment and start of manual/automated cleaning.
Detergent: A water-soluble cleansing agent which combines with impurities and dirt to make them more soluble; differs from soap in not forming a scum with the salts in hard water.
Log reduction: A log reduction reduces the number of microorganisms by 10X.
Enzymes: Proteins that act as catalysts to bring about specific biochemical reactions.
Medical-grade compressed air: Air supplied from cylinders, bulk containers or medical air compressors, or reconstituted from oxygen USP and oil-free dry nitrogen NF
Non-enzymatic surfactant: Chemicals that break the cohesive bond between soil and a surface; can be anionic, neutral (non-ionic) or cationic. A common type of cationic surfactant is quaternary ammonium.
pH: Number denoting alkalinity or acidity The pH scale is logarithmic and runs from 0 to 14; the neutral point is 7. Numbers below 7.0 indicate acidity, and those above 7.0 indicate alkalinity.
Potable water: Water that has been treated and delivered in a manner so that it meets EPA guidelines as suitable for drinking.
Reverse osmosis water: Water that has been processed to reduce impurities. Processing methods include filtration, deionization, distillation, or reverse osmosis (RO), either singly or in combination.
Surfactant: Decreases surface tension to penetrate the soil. Surfactants contain emulsifying properties which prevent debris from re-depositing on instruments.
Definitions are from AAMI ST:91, 2015, AORN Recommended Guidelines 2017, and the CDC.

