eLearning Self-paced
Understanding Barrett's Esophagus
Overview
Guide to Study
eLearning Activity
Who is Barrett? How did he happen to have a condition named for him? Read on to learn more.

Norman Barrett was born in Australia in 1903. He was educated in England, at Eton and Trinity College, Cambridge. Within 3 years of graduation, Barrett was elected to fellowship of the Royal College of Surgeons and had been awarded the postgraduate degree Master of Surgery. Supported by a Rockefeller Travelling Fellowship, Barrett studied in the United States in 1935 and 1936. Barrett went to America with the intent of studying upper gastrointestinal surgery, but he was urged by Donald C. Balfour at the Mayo clinic to enter into a newer field with more opportunities. That is when he settled on thoracic surgery.
In 1950, Professor Norman Barrett noticed that, in certain patients, the distal esophagus had a lining that looked just like the lining of the stomach. That lining is called columnar epithelium. He originally believed that some people were born with this condition and described it “as tubular portion of the stomach being trapped in the chest”. Over time, other physicians argued that this stomach tissue was not stomach at all, but esophagus. It was suggested that these areas of stomach tissue on the lining of the esophagus be called “Barrett’s Ulcers”. Norman Barrett further described this condition as “lower esophagus lined by columnar epithelium”. Ever since, this disease described by Norman Barrett became known as “Barrett’s Esophagus”(BE).
So what is Barrett’s Esophagus? It is a condition referring to an abnormal change (metaplasia) in the cells of the distal portion of the esophagus. It is the replacement of a portion of the normal stratified squamous epithelium with columnar epithelium with goblet cells, which are usually found lower in the gastrointestinal tract. Barrett’s may also be referred to as intestinal metaplasia, or IM. It is further classified as low grade or high-grade dysplasia. Barrett’s is a precursor to esophageal cancer. It can can be readily seen on an esophagogastroduodenoscopy (EGD) or upper endoscopy, but many people who have reflux may have never had an EGD.
The following graphics depict an anatomic visualization and an endoscopic view.
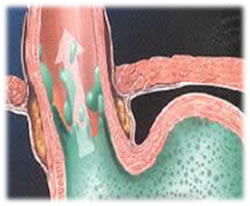
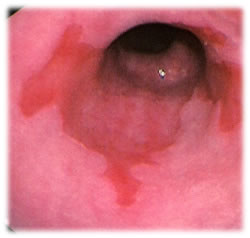
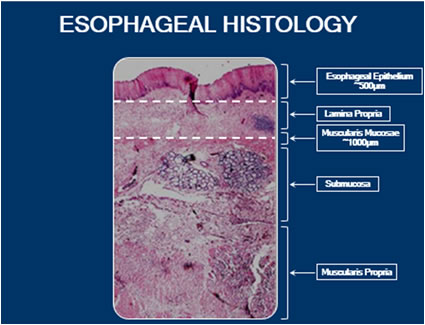
There are five layers to the esophageal lining:
- The top layer is the Esophageal Epithelium (EP) and is about ½ millimeter in depth. This is the layer that changes to intestinal metaplasia when someone has Barrett’s. Barrett’s is approximately ½ millimeter in depth.
- The next layer is the Lamina Propria (LP). This is the support structure to the esophageal epithelium. If Barrett’s progresses to cancer, we see intramucosal cancers in the Lamina Propria.
- The next layer is the Muscularis Mucosa (MM). These three layers (EP, LP, and MM) make up the Esophageal Mucosa. The total depth of these three layers is approximately 1 millimeter. This depth is important when understanding the treatment of the disease, as this should be the area to treat when removing all of Barrett’s tissue.
- The next layer is the Sub Mucosa (SM). If any therapies encroach into this layer, it raises concern for complications. This will become apparent when discussing the treatment therapies for Barrett’s.
- The last layer is the Muscularis Propria (MP), which is the outer muscle layer of the esophagus. If an esophagus has to be removed or resected, it is to this level.
Did you know:
The five layers of the esophageal lining are:
- Esophageal Epithelium
- Lamina Propria
- Muscularis Mucosa
- Submucosa
- Muscularis Propria
Before we can understand Barrett’s Esophagus classifications, it is important to get a better understanding of Barrett’s most common risk factor, its precursor: Gastroesophageal Reflux Disease (GERD). Recent studies suggest that GERD is increasing in prevalence. The diagnosis of GERD is associated with a 10-15% risk of Barrett’s esophagus .BE has been detected in ~15% of patients with chronic GERD.
GERD is a digestive disorder that involves the Lower esophageal sphincter. The lower esophageal sphincter is designed to prevent the flow of contents from the stomach back up into the esophagus. Sometimes there is a physical or mechanical defect that allows stomach contents to reflux into the esophagus, as depicted by the arrow in the graphic below. This refluxate can include stomach acids, which is what causes the burning sensation we call “heartburn”.

This may also cause regurgitation, chronic cough and sore throat, difficulty sleeping, perhaps even asthma, as the patient can aspirate some of the refluxate. Esophagitis is an inflammation of the esophagus in response to reflux. Over time, it can erode the lining of the esophagus, causing ulcers and dysphagia, or difficulty swallowing.
GERD is often treated with different medications. Over the counter medications, like Tums or Rolaids, might give some relief. A physician may also prescribe a Proton Pump Inhibitor which reduces stomach acid production. Some of these can be obtained over the counter as well. Examples include Nexium, Prilosec or Prevacid. Patients who suffer from chronic heartburn (weekly) should be evaluated by a GI doctor. Over the counter medications rarely cure Barrett’s; they only address the reflux.
It is important to note the different stages of Barrett’s. Just looking at Barrett’s tissue (through the endoscope), Intestinal Metaplasia (IM) with or without dysplasia cannot be determined endoscopically. Physicians take multiple biopsies with the intent of confirming Barrett’s and sampling any dysplasia that might be present. It is based on these samples that pathologists try to determine the grade.
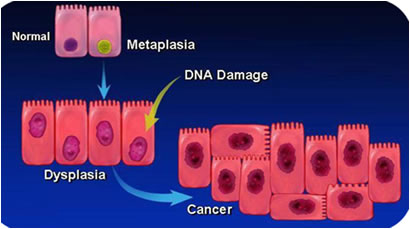
The stages, or grades, of Barrett’s are: Non-dysplastic, Indefinite, Low grade Dysplasia, and High Grade Dysplasia, which can lead to Intramucosal Carcinoma. So let’s break down what we see in the preceding image:
- The graphic representation shows a normal nucleus cell, next to a green Intestinal metaplasia nucleus cell, which has already started to change in its genetic makeup (depicted in green, it is starting to get sick). This gives it a growth advantage. On histology, the structures of the two cells look the same, but pathologist can’t tell the genetic makeup of what is on the inside. It is just percolating away with its genetic change.
- By the time dysplasia is noted, its nucleus structure is different, as represented by the nuclear changes. The nucleus has become larger and darker and has been able to divide and expand over the mucosa.
- It is also possible that when dysplasia is detected, some abnormal nuclei and the cells themselves are chaotic and may have undergone transformation to carcinoma.
The important takeaway is that the genetic changes to a cell precede detection of dysplasia. It can be challenging to accurately classify Barrett’s grades based on biopsy samples. Not all pathologists diagnose Barrett’s exactly the same way using the same set of criteria. In several studies, it was found that if a tissue sample is given to two different pathologists, they may have different interpretations 85% of the time, so agreement is often moderate or poor. This is called pathology discordance. Additionally, in another study, there was ~30% discordance between expert GI pathologists on agreement to baseline disease grade. Even with diagnosis on non-dysplastic IM (the least severe form of BE), the fact is an increase and accumulation of DNA nucleus abnormalities can precede and drive intestinal metaplasia progression to likelihood of a more severe form of low or high-grade dysplasia.
Did you know: Treatment of GERD with over-the-counter medications can sometimes mask the development of Barrett’s Esophagus.
Even with a diagnosis of non-dysplastic intestinal metaplasia, the possibility exists for an increase and accumulation of DNA nucleus abnormalities. This can drive intestinal metaplasia progression to likelihood of a more severe form of low- or high-grade dysplasia.
The first image reveals a histology slide of non-dysplastic tissue, along with histograms that represent the number of changed DNA nucleus cells involved with this diagnosis. The progression of intestinal metaplasia to esophageal adenocarcinoma is 0.5% per year.
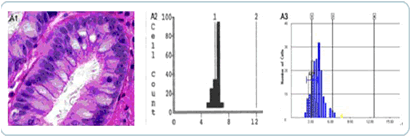
Figure 1: Non-dysplastic
Figure 2 shows the next level, showing the histology slide of low-grade dysplasia. The histograms show more DNA nucleus changes and accumulation involvement. The progression of intestine metaplasia to low-grade dysplasia is 4.0% per patient per year. Consequentially, the progression from LGD to esophageal adenocarcinoma is~0.7% per year.
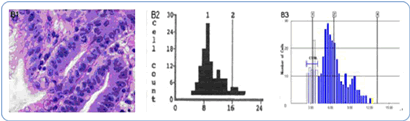
Figure 2: Low-grade dysplastic
The histology slide in Figure 3 depicts high-grade dysplasia, with histograms showing even more DNA nucleus changes and accumulation.
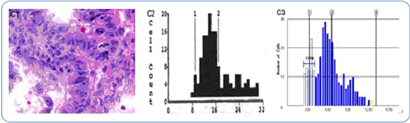
Figure 3: High-grade dysplastic
Figure 4 reveals the final stage, with a diagnosis of esophageal adenocarcinoma. The histology slide and histograms reveal the worst DNA nucleus abnormalities. Progression from HGD to CA is ~7% per year.
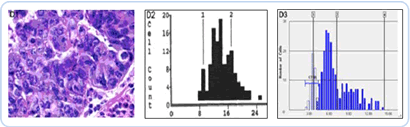
Figure 4: Esophageal adenocarcinoma
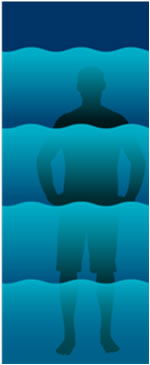 The grading of Barrett’s Esophagus can be analogous to a person wading out into the ocean:
The grading of Barrett’s Esophagus can be analogous to a person wading out into the ocean:
Visualize a man walking into the shallow water as the waves barely touch his ankles. This represents a non-dysplastic diagnosis.
As he goes deeper into the ocean, the water is about knee level. This symbolizes a diagnosis of low grade dysplasia.
As he wades out to chest-level water, the diagnosis is high level dysplasia.
Going deeper and deeper, the water goes over his head and he is being swept out into the sea. This represents the possibility of high-grade dysplasia progressing to cancer (esophageal adenocarcinoma).
The photos show the anatomical changes seen in the progression of acid reflux to Barrett’s to adenocarcinoma.
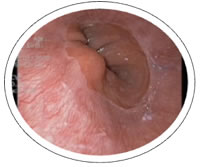
The above image shows normal squamous tissue which, when paired with the chronic insult of acid, may lead to chronic inflammation.
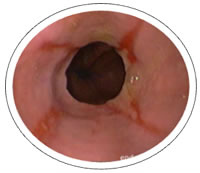
The second image shows esophagitis.
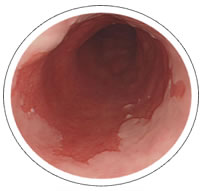
The third image reveals Barrett’s Esophagus. Genetic/DNA changes occur in Barrett’s tissue and these changes accumulate over time. The growth pattern of these precancerous cells can be subtle. People with Barrett's esophagus are 30 to 125 times more likely to develop cancer of the esophagus than the general population.
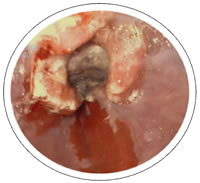
The fourth image shows Barrett’s Esophagus which has progressed to esophageal adenocarcinoma.
Did you know: People with Barrett's esophagus are 30 to 125 times more likely to develop cancer of the esophagus
Progression Rate
Statistics show that the majority of patients have non-dysplastic Barrett’s and the risk of progression appears to be very low at 2.9% over 10 years However, it is important to put some of these progression rates into perspective: in other parts of the body, this level of progression risk is cause enough for intervention.
The Standard of Care calls for the removal of Pre-Cancerous Lesions in these other areas:
- cervical dysplasia
- breast masses
- dermatologic lesions (moles, actinic keratoses)
- colorectal
Consider management of colon polyps: Colon polyps are typically removed, reducing the chance of the development of colorectal cancer. A 1 cm adenomatous colon polyp progresses to cancer at the rate of 0.5% per patient per year, the same rate as Barrett’s progression but we treat these diseases very differently. Polyps are removed, yet some practitioners will still maintain surveillance for progression of early Barrett’s before moving to intervention.
According to the American Cancer Society(2020), the rates of esophageal cancer in the United States has been fairly stable for many years, but over the past decade they have been decreasing slightly. (GI society reports vary on this statement, so further investigation of the data continues.) Esophageal cancer makes up about 1% of all cancers diagnosed in the United States, but it is much more common in some other parts of the world, such as Iran, northern China, India, and southern Africa.
Although many people with esophageal cancer will go on to die from this disease, treatment has improved, and survival rates are getting better. During the 1960s and 1970s, only about 5% of patients survived at least 5 years after being diagnosed. Now, about 20% of patients survive at least 5 years after diagnosis. This number includes patients with all stages of esophageal cancer. Survival rates for people with early stage cancer are higher.
Barrett’s esophagus has been detected in ≈15% of patients with chronic GERD (> 5 years); advancing age (>50 years) is also a risk factor. Additional factors such as Caucasian race, male gender, obesity and tobacco usage also come into play. Family history is important: BE is more common in first-degree relatives with known BE. Interestingly, alcohol consumption does not increase risk of BE. In fact, drinking wine may be a protective factor.
Since the relationship between Gastroesophageal Reflux Disease and Barrett’s is so strong, let’s take a quick look at the incidence of GERD in the U.S. population:
- 44% report monthly symptoms of GERD
- 18% report weekly symptoms of GERD
- 7% report daily GERD
- 1/3 of patients with GERD may go on to develop Erosive Esophagitis. With a diagnosis of esophagitis, the cell structure is changing but can be corrected with prescriptions for heartburn
According to the American College of Gastroenterologists (2018), patients with Barrett’s esophagus have a low risk of esophageal cancer. The overall risk of cancer may increase as the years go by, but more than 90% of people with Barrett’s esophagus WILL NOT develop cancer. Therefore, Barrett’s esophagus is a condition that needs to be addressed, even though the vast majority of patients with Barrett’s will never get cancer. People who experience weekly heartburn or acid regurgitation are 64 times more likely to get esophageal adenocarcinoma than people who have never experienced these symptoms, 40% of people with esophageal adenocarcinoma deny ever experiencing heartburn. Why these people developed esophageal adenocarcinoma remains a mystery. This would indicate that only screening people with GERD-type symptoms would miss a significant portion of those affected. Although many Barrett’s patients may have the obvious signs of reflux, some patients with Barrett’s never exhibit any symptoms.
It is unknown why, but GERD affects men more than women. Most patients are white males with a mean age of 55 to 65 years. Men are 3 to 4 times more likely to have Barrett’s esophagus compared to women. Caucasians are about 10 times more likely to have Barrett’s esophagus than persons of African American ethnic background. Thirteen percent of men over age 50 with chronic reflux will go on to develop Barrett’s. The lowest published rate of adults affected by Barrett’s is 3.3 million people. However, more recent publications show the rates to be even higher at 7.8 to 11.2 million adults.
Barrett’s Esophagus can only be diagnosed with an Upper GI endoscopy and biopsy
Did you know: Without an endoscopy, it is not yet possible to distinguish GERD patients with Barrett’s Esophagus from those in whom Barrett’s Esophagus is not present.
Screening
The goal of a screening and surveillance program for BE is to identify individuals at risk for progression to esophageal adenocarcinoma
- Screening for BE may be considered in men with chronic (>5 years) and/or frequent (weekly or more) symptoms of gastroesophageal reflux (heartburn or acid regurgitation) and two or more risk factors for BE or Esophageal Adenocarcinoma (EAC). These risk factors include: age >50 years, Caucasian race, presence of central obesity, current or past history of smoking, and a confirmed family history of BE or EAC (in a first-degree relative)
- Given the substantially lower risk of EAC in females with chronic GER symptoms (when compared with males), screening for BE in females is not recommended. However, screening could be considered in individual cases as determined by the presence of multiple risk factors for BE or EAC (age >50 years, Caucasian race, chronic and/or frequent GERD, central obesity, current or past history of smoking, and a confirmed family history of BE or EAC (in a first-degree relative))
- Screening of the general population is not recommended
The good news about Barrett’s Esophagus is that it can be treated. The options for management and eradication of Barrett’s have evolved over the years, and physician practice habits are changing as more options become available. Surveillance and medical management have been the pre-dominant management strategies for Barrett’s for years. But these strategies were only applicable to the earliest forms of Barrett’s and were focused more on catching the progression to worsening grades. The morbidity associated with surgery encouraged the development of endoscopic techniques for Barrett’s removal, both through resection and ablation. In recent years, new endoscopic therapies have emerged that are successfully treating the disease before it progresses to cancer. As these technologies have proven themselves effective and safe, their use in the management of Barrett’s has grown significantly over the past several years
Surveillance
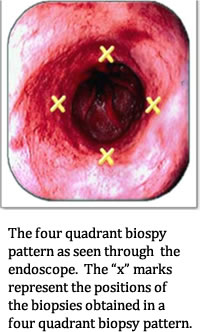
In the practice of surveillance, the physician performs an endoscopy and takes samples of the suspected Barrett’s tissue. Physicians are encouraged to use the “Seattle Protocol”, which requires biopsy samples every 1 cm to 2 cm in 4 quadrants. So if there was a 4 cm section of Barrett’s, that would be up to 16 biopsies.
According to the ACG guidelines (2018), in patients with suspected BE, at least 8 random biopsies should be obtained to maximize the yield of IM on histology.In patients with short (1–2 cm) segments of suspected BE in whom 8 biopsies are unattainable, at least 4 biopsies per cm of circumferential BE, and one biopsy per cm in tongues of BE, should be taken.
In patients with suspected BE and lack of IM on histology, a repeat endoscopy should be considered in 1–2 years of time to rule out BE.
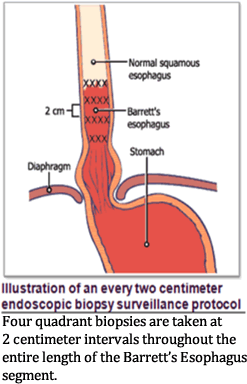
The length of time between surveillance procedures varies and is largely up to the physician. The ACG (2018) recommends the following surveillance protocol:
- For BE patients without dysplasia, endoscopic surveillance should take place at intervals of 3 to 5 years
- Patients diagnosed with BE on initial examination do not require a repeat endoscopy in 1 year for dysplasia surveillance
- For patients with indefinite for dysplasia, a repeat endoscopy after optimization of acid suppressive medications for 3–6 months should be performed. If the indefinite for dysplasia reading is confirmed on this examination, a surveillance interval of 12 months is recommended
- For patients with confirmed low-grade dysplasia and without life-limiting comorbidity, endoscopic therapy is considered as the preferred treatment modality, although endoscopic surveillance every 12 months is an acceptable alternative
- Patients with BE and confirmed high-grade dysplasia should be managed with endoscopic therapy unless they have life-limiting comorbidity
There are some limitations to a managing Barrett’s with surveillance. Patient compliance is a major factor in surveillance intervals: When something doesn’t hurt or bother you, it’s easy to “forget” your next endoscopy. The technique only monitors the Barrett’s, looking for progression to a more severe grade. This has been called out as a contributing factor to patient anxiety. Imagine having a pre-cancerous lesion managed by a biopsy every 3-5 years.
Biopsy
According the ACG (2018), endoscopic surveillance has numerous shortcomings. Dysplasia may not be visible endoscopically and the distribution of dysplasia and cancer is highly variable. Even the most thorough biopsy surveillance programs have the potential for sampling error. Current surveillance programs are expensive and time consuming
In some cases, endoscopically normal appearing squamous tissue may grow on top of Barrett's lining. When this occurs, the Barrett's lining is not only still there, but looks like normal squamous lining through the endoscope. This can lead one to think that the Barrett's is gone when it is simply buried beneath normal appearing squamous lining. This is often referred to as SSIM (subsquamous intestinal metaplasia) or buried glands.
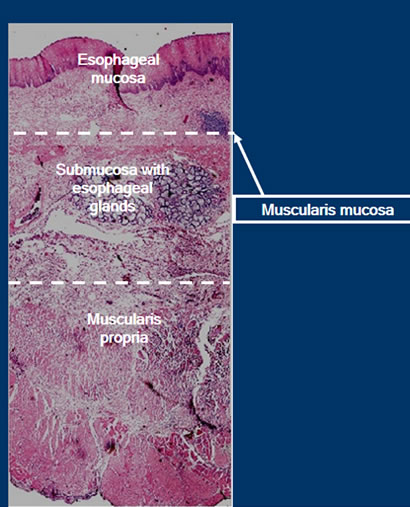
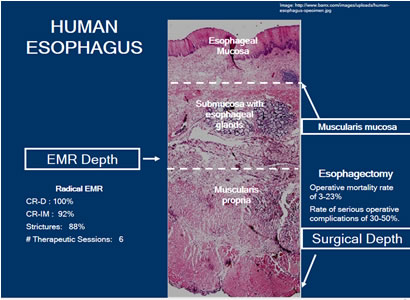
A biopsy of the human esophagus takes place at the depth of the lamina propria/muscularis mucosa layer, as illustrated in the preceding figure. The depth is adequate for tissue acquisition to send to pathology, but there are some issues to consider. Biopsies are inconsistent in terms of where they are obtained along the esophagus.
Sampling error refers to a situation where pathology exists but is missed because the clinician did not choose that area to sample. You can endoscopically see Intestinal Metaplasia (IM), but there may be an advanced grade of Barrett’s in an area that was not sampled. There is a risk that surveillance biopsy may have missed tissue with a more severe diagnosis.
Enhanced Imaging
Enhanced imaging may provide better detection. Techniques such as narrow band imaging (NBI) and chromoendoscopy show promise for better detection of dysplasia in Barrett’s esophagus. In fact, clinicians have reported numerous advantages using these new, advanced techniques:
- Changes in mucosal and vascular patterns can be identified which can predict histology indicative of dysplasia, resulting in a potentially improved ability to screen for Barret's Esophagus, detect dysplasia in established Barrett’s Esophagus and detect recurrent dysplasia/neoplasia after ablation.
- Abnormal mucosal/vascular patterns reliably mark advanced dysplasia and early esophageal adenocarcinoma.
- When used with targeted biopsy, these techniques may reduce dependency on random biopsies.
- Fewer biopsies per patient and higher proportion of dysplasia identified, compared to standard white light endoscopy.
Diagnostic yield for dysplasia or cancer increased by 34% when NBI added, when compared to White Light Endoscopy with random biopsies.
Narrow band imaging (NBI) is a high-resolution endoscopic technique that enhances the fine structure of the mucosal surface without the use of dyes.
Chromoendoscopy involves the topical application of stains or pigments to improve tissue localization, characterization, or diagnosis during endoscopy. The stains used for chromoendoscopy are transient, unlike the stains used to tattoo lesions, and the equipment needed for chromoendoscopy is widely available. These imaging techniques are simple, quick, inexpensive and safe.
Surgery-Esophagectomy
There are instances where the complete resection of Barrett’s is necessary. Esophagectomy has been an option that has been reserved for patients with high-grade dysplasia and cancer. Surgical depth is down to the Muscularis Propria. In the hands of an experienced esophageal surgeon who performs these surgeries in a center experienced in the care of patients undergoing esophagectomy, the mortality rate is around 1-8%. On the other hand, the surgical mortality in low volume centers is in the range of 16-23%.
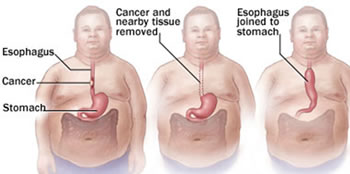
The rate of serious complication ranges from 30-50% post esophagectomy, resulting in a poor quality of life for the patients. These can include post-operative complications such as esophageal anastomotic leak, pneumonia, deep vein thrombosis, gastric necrosis, cardiac arrhythmias, wound infections, prolonged ileus (lack of bowel function), GI bleeding, stenosis and diarrhea.
Endoscopic Mucosal Resection (EMR)
Endoscopic Mucosal Resection, or EMR, is the process of endoscopic resection of Barrett’s lesions. EMR is often used for raised lesions or advanced disease. These are often referred to as “nodules”. Physicians may choose EMR if they suspect there is already a cancer in a suspicious area, or if they would like a larger area for histological samples. It is an excellent alternative to esophagectomy when you have nodularity or small focal areas of advanced disease because it preserves the esophagus. EMR can be done as a primary treatment for the focal lesion. It can be complemented by other therapies for the larger, flat areas of disease.
There are several techniques used for EMR; the two common techniques will be explored here.
Technique One: This is done by using a small cap with a small wire loop that fits on the end of the endoscope. The nodule is suctioned into the cap and the wire loop is closed while cautery is applied.

Step 1: Injection of target lesion.
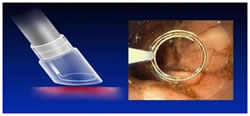
Step 2: Positioning the snare.
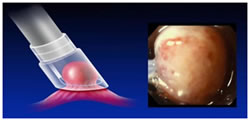
Step 3: Suction & snare of lesion.
Technique Two: This is done by using a small ligation band, followed by a cautery loop. The cautery loop is around the nodule and energy is applied. Once the nodule is released from the mucosal wall, it is retrieved in the usual fashion.
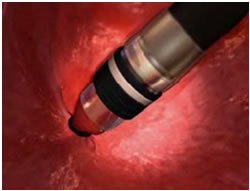
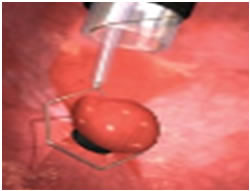
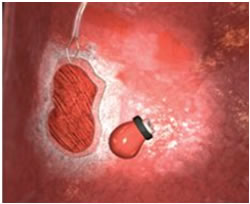
EMR is very successful in removing large areas of tissue. It enables evaluation of changes in diseased tissue and can be used to obtain large biopsies for diagnosis and local tumor staging. Frequently, EMR reveals more advanced tumor stages. It is often recommended in combination with additional ablation techniques.
EMR does have some limitations. Because the resecting is down to the level of the submucosa, there is a risk for strictures. When injury is caused to the submucosa, there is a risk of stricture formation. This can limit its utilization to advanced disease, so often focal EMR is complemented with an ablation technique in a broad field of flat disease.
EMR/Mucosectomy target depth is into the submucosa. This preserves the esophagus, while removing a substantial piece of tissue. One technique is to remove all of the Barrett’s tissue through a Stepwise Radical Endoscopic Resection (SRER). SRER is a technique in which the complete Barrett’s Esophagus segment is removed in consecutive endoscopic resection sessions. Retrieval of the entire Barrett’s Esophagus segment for histological assessment is ideal, as it may permit referral to surgery for advanced lesions. Single-center studies have shown excellent results of SRER for HGD or early cancer. An important limitation of SRER is the rate of complications, such as esophageal stenosis requiring dilation. Focal EMR is when there is only a resection of certain areas. EMR is successful at removing these focal areas, but there still is the risk of scarring and the potential for strictures.
Argon Plasma Coagulation (APC)
Argon-plasma coagulation (APC) is a widely used, contact-free, operator dependent endoscopic ablation technique for the eradication of BE with the relevant risk of stricture formation and buried metaplastic glands under a layer of neosquamous epithelium. Hybrid APC, which combines APC with submucosal saline injection, was developed to address these complications. The Barrett epithelium is lifted with a 0.9% saline injection using a high-pressure water jet, creating a safety cushion under the mucosa. The Barrett esophagus can then be ablated more thoroughly and with a higher energy setting, without an increase in side effects or complications.
In a pilot study of 50 patients conducted by H. Manner et.al (2015), Hybrid-APC was found effective and safe for BE ablation in a tertiary referral center. The rate of stricture formation was only 2%, compared with a 5-10% stricture formation after thermal ablation. Further studies are required to confirm the present results.
There is a certain user variability as it related to the duration of the application and how close to place the tip to the mucosa in order to be effective. This affects the depth of the burn, and recall that Barrett’s lives in the ½ millimeter range. The optimum ablation depth is 1mm. Focal APC does not have a mechanism to control the depth of burn, so the depth can be variable based on the user application. This includes how close the catheter is placed to the tissue and how long the energy is applied.
Photodynamic Therapy (PDT)
PDT, or Photodynamic Therapy, is a treatment that is used as an alternative to surgery in non-surgical candidates for the treatment of high-grade dysplasia and early cancers. It was the first medical technology that demonstrated a decrease in progression rates to cancer in HGD patients. It can also be used to reduce the tumor mass in patients with advanced esophageal cancer.
PDT Technique:
- PDT uses a combination of photosensitizer--a light-activated drug-- and laser light to destroy abnormal cells. PDT patients are injected with a photosensitizer to render their tissue extremely sensitive to laser light.
- The lesion is then illuminated with a laser light of proper power and wavelength, or color.
- The interaction of laser light and the photosensitizer causes a chemical reaction, killing the abnormal cells.
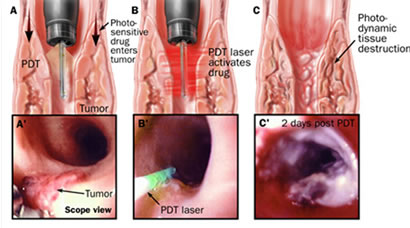
PDT tends to affect the esophagus to the submucosal layer. While effective, it can produce some complications. Results vary in the 51-77% response rate. In studies the application was not circumferential, and in some instances, exceeded well into the submucosal tissue, and in others, not deep enough into the epithelium.
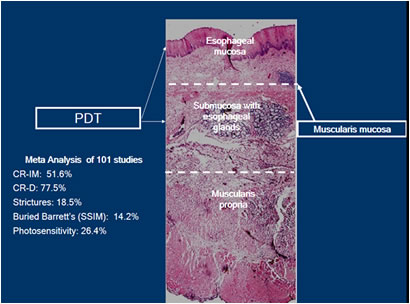
Although PDT was the first therapy to show significant decrease in high grade dysplasia and cancer in patients with BE, its use has been limited, primarily because of its costs and its complications:
- Photosensitivity (can persist from 30-90 days after injection with the photosynthesizing drug)
- Strictures (tend to occur in about one third of patients treated, and normally happen 3 to 4 weeks after therapy)
- Subsquamous Barrett’s mucosa (buried Barrett’s can be found when the Barrett’s mucosa is not eliminated by therapy and is overgrown by surrounding squamous mucosa)
- Chest pain
- Nausea
- Vomiting
Cryotherapy
By freezing tissues to extreme cold temperatures, cryotherapy attempts to remove abnormal cells and allow for the re-growth of new, healthy cells in their place. Cryotherapy has been used in patients with Barrett’s esophagus with high-grade dysplasia and persistent low-grade dysplasia as well as early stage esophageal cancer that is not amenable to standard therapies, including surgery, chemotherapy and radiation therapy. There are two different cryogens. One is a rapid flow CO2 stored at room temperature as a cryogen source and a catheter to deliver the cryogen to the treatment site. By utilizing the Joule-Thompson effect, the CO2 expands and rapidly cools to -78C as it exits the catheter. Another is a system that transports low-pressure liquid nitrogen through a specially designed catheter that is passed through a standard endoscope. This freezes the desired tissue. It utilizes large volumes of gas, which requires a second catheter to be placed so it can continuously remove the gas.
It is reported that three to eight treatments are necessary in order to see any measurable results. There is a lack of substantial data that enables researchers to conclude if depth of ablation is sufficient, but the current data suggests that it’s around 1mm to 2mm in depth. Because Cryotherapy is also uncontrolled and user-dependent those depths can vary significantly.
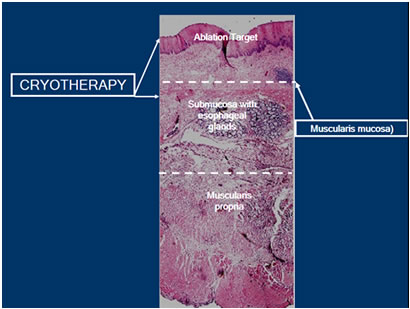
As an effective treatment modality, cryotherapy has distinct advantages and disadvantages. One advantage is that it is through the scope. One important limitation to cryotherapy is that there is no visual endpoint that lets you know when enough treatment has been performed. So it’s up to the user to decide when to stop the application of the therapy. This would account for uncontrolled depth of ablation.
Radiofrequency Ablation
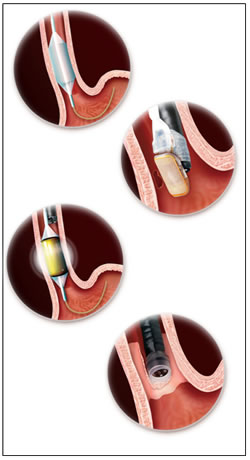 Radiofrequency Ablation is commonly referred to as RFA. RFA is indicated for ablation of Barrett’s esophagus, which may include non-nodular, ND-IM, LGD and HGD.
Radiofrequency Ablation is commonly referred to as RFA. RFA is indicated for ablation of Barrett’s esophagus, which may include non-nodular, ND-IM, LGD and HGD.
RFA has a few key treatment goals, one of which is the delivery of ablative energy in less than one second, allowing long or short segments of BE to be treated quickly. It provides a consistent application of bipolar energy that uniformly removes the esophageal epithelium. It is a contact probe, in contrast to other therapies that are non-contact. RFA can be done circumferentially with a balloon based system for long segment BE or a more focal device ffor short segment or islands of BE. .
RFA’s controlled treatment depth of less than 1 millimeter (or to the Muscularis Mucosae) reduces the risk of stricture formation. This also reduces the potential for buried glands and improves patient tolerability.
After Barrett’s is confirmed by a pathologist based on a prior EGD, the patient is scheduled for ablation.
During the ablation procedure, the distal and proximal margins of the disease are noted
For long segment Barrett’s (3cm), the physician introduces an ablation balloon over a guide wire and positions it in the area to be ablated. A special generator then delivers the energy to the balloon. This area is cleaned, and the ablation steps are repeated. It is a process designed to insure a uniform ablation depth and to minimize complications.
To treat short segment or islands of Barrett’s, a focal catheter is mounted on the tip of the scope to deliver the energy. Each area is ablated twice.
As you review the following graphic, note that the ablation is to the muscularis mucosa. This is deep enough to eliminate the columnar epithelium and underlying lamina propria. Typically, buried glands will be present in these layers, so they are ablated simultaneously. When the Barrett’s tissue is eliminated, normal squamous epithelium grows back in its place.
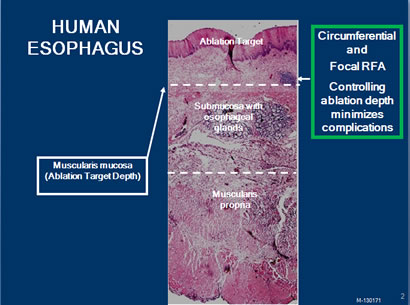
There is a significant amount of research that has gone into the study of RFA to ensure that it consistently works and remains safe:
Long term data
- RFA resulted in complete eradication of disease in 98% of NDBE patients, with 2.5-years follow-up.
- At 5 years, 92% of patients maintained durable cure, and no patients demonstrated neoplastic progression.
Rigorous Randomized control trial for Dysplasia published in the New England Journal of Medicine
- Complete Response Barrett’s: 83%
- Complete response Low Grade Dysplasia: 95%
- Complete response High Dysplasia: 90%
Compared to a diagnostic EGD, an RFA procedure is therapeutic, therefore takes longer. Multiple intubations extend the procedure time and can affect the patient with a sore throat lasting a day or so. Possible complications may include mucosal laceration, acute bleeding and esophageal stricture. The delivery of the energy is automated, so it eliminates the user variability and the duration of the application of therapy. This controls the depth of ablation, insuring effectiveness while limiting complications. With 68+ publications to date, it is the most well-research Barrett’s ablation device available today. The studies consistently show a 90+% efficacy rate, which means complete elimination of all Barrett’s tissue.
Goals of Treatment
- Eliminating the entire at risk mucosa
- Minimizing complications
- Minimizing recurrence, strictures and bleeding
- Decreasing the need for frequent surveillance
To meet these objectives, the correct depth ablation should target to, but not beyond, the muscularis mucosa. This is desired to eliminate all intestinal metaplasia, but also to avoid complications.
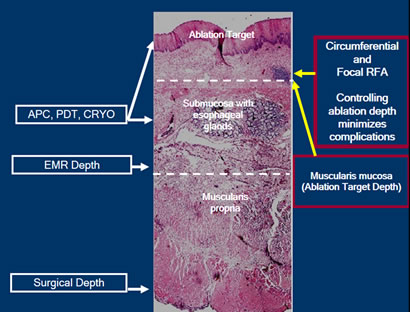
Focal APC is uncontrolled, and no studies have documented a consistent depth of burn. PDT application is effective, but associated complications and inconsistent efficacy limit its widespread utilization. Cryotherapy data is not substantial to date to recognize if depth of ablation is sufficient. However, data suggest that it’s around 1-2 mm in depth. EMR has a role when early cancers or Barrett’s tissue extends into the submucosa. Stricturing tends to limit its utilization to Barrett’s nodules.
Society Guidelines influence many physicians’ practice patterns. The American College of Gastroenterologists (26), the American Gastrointestinal Association (3,28) , the American Society for Gastrointestinal Endoscopy (6) and the Society of American Gastrointestinal and Endoscopic Surgeons (24) have statements and guidelines related to the management of Barrett’s Esophagus.
For your convenience, the guidelines are listed in the suggested readings section.
Conclusion
Regardless of the treatment options used in an individual facility, it is important for endoscopy nurses to incorporate knowledge of various factors into their practice. These factors, which will enhance care of the Barrett’s Esophagus patient, include knowledge of esophageal anatomy, precursors and disease progression of Barrett’s Esophagus and knowledge of available treatment options.
CNE Certificate Process
Note: Your computer should be connected to a printer before completing the Post-Testing in order to permit printing your course certificate.

