eLearning Self-paced
Understand Endoscopic Ultrasound and Fine Needle Aspiration
Overview
Guide to Study
eLearning Activity
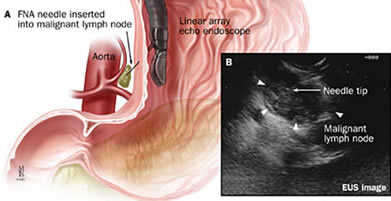
Since its initial report of use in 1992 as an imaging modality, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) has been incorporated into the diagnostic and staging algorithm for the evaluation of benign and malignant diseases of the gastrointestinal tract and adjacent organs. This constitutes a major breakthrough in the endoscopic field, which has gradually transformed EUS from a pure imaging modality into a more interventional procedure. EUS permits the collecting of samples, providing definitive cytological and histological evidence of malignancy, which has strongly contributed to changing EUS from a subjective, operator-dependent procedure into a more objective one.
EUS is a technique that combines and modifies techniques of gastrointestinal endoscopy and ultrasonography. It uses sound waves known as ultrasound during an endoscopic procedure to look at or through the wall of the gastrointestinal tract. The common indications for EUS-TA (tissue acquisition) include diagnosis and staging of pancreaticobiliary, esophageal, gastric and rectal malignancies and lung cancer, along with evaluation of GI subepithelial lesions and lymphadenopathy. In addition, EUS-TA can be used for numerous nonmalignant processes and mediastinal lesions such as tuberculosis, sarcoidosis, abscesses and cysts.
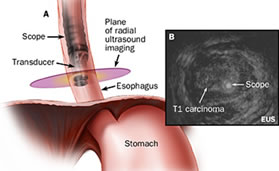
EUS is a diagnostic modality. The concept behind endoscopic ultrasound is to diminish the distance between the ultrasonic source and the organs to be imaged. An ultrasound transducer is incorporated into the tip of an endoscope. While endoscopically visualizing the lumen, the probe can be guided and placed adjacent to the area of disease, thus avoiding bones, tissue and air-filled structures, all of which limit sound wave imaging clarity.
The short distance between the ultrasonic source and the target permits the use of high frequency, high resolution sound waves which, due to their shorter penetration depth, are not suitable for transcutaneous ultrasonography.
Finding an abnormality with regular endoscopy is like seeing the tip of an iceberg. Endoscopy shows only the inner surface of the digestive tract and cannot show the abnormality beyond the visible surface. But in the same way a ship’s sonar can depict the whole iceberg under water, the high frequency of EUS reveals the full extent and nature of abnormalities, including information that is critical to accurate diagnosis and optimum care. This allows physicians to see organs and structures not typically visible during endoscopy alone, such as the layers of the gastrointestinal tract wall, the liver, pancreas, lymph nodes and bile ducts.
Echogenicity of the tissue refers to the ability to reflect or transmit US waves in the context of surrounding tissues. Whenever there is an interface of structures with different echogenicities, a visible difference in contrast will be apparent on the screen. Based on echogenicity, a structure can be characterized as anechoic, hypoechoic and hyperechoic.
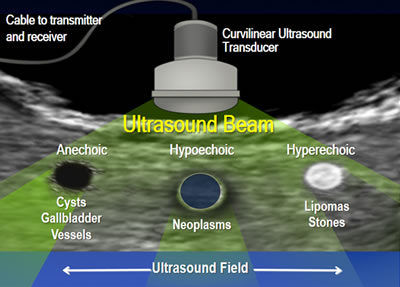
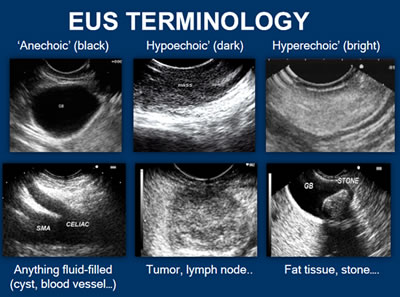
- Anechoic: Structures appear black, meaning no internal echoes. Examples include cysts, vessels, gallbladder ascites and water.
- Hypoechoic: Gives off fewer echoes; they are darker than surrounding structures. Examples include lymph nodes and tumors.
- Hyperechoic: Increased density of sound waves compared to surrounding structures. Examples include bone and fat calcifications.
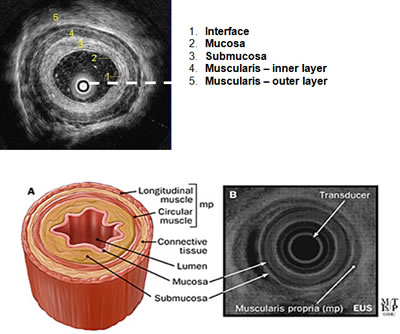
Take a look at the example of a cross-section of the esophagus. On endoscopic ultrasound (radial), the gastrointestinal wall appears as five layers:
- The first layer is hyperechoic and corresponds to the superficial mucosa.
- The second layer is hypoechoic and corresponds to the deep mucosa.
- The third layer is hyperechoic and corresponds to the submucosa plus the acoustical interface between the submucosa and the muscularis propria.
- The fourth layer is hypoechoic and corresponds to the muscularis propria minus the acoustical interface between the submucosa and the musclaris propria.
- The fifth layer, the serosa and subserosal fat, is hyperechoic.
The scope used for EUS is similar to a regular endoscope with the added component of an ultrasound transducer. Endoscopic ultrasonography is the most sensitive imaging tool for the diagnosis of chronic pancreatitis, and has been proven to be more accurate than the CT scan. Endoscopic ultrasound is a highly technical, low-risk diagnostic procedure that utilizes high-frequency ultrasound during endoscopy to evaluate and diagnose digestive tract disorders. EUS allows imaging of the pancreas at close proximity with high resolution. Hence, it may detect changes consistent with chronic pancreatitis in the patient in whom ERCP and other tests are normal. An EUS scope is advanced within the gastrointestinal tract against, or in close proximity to, the pancreas. From a position in the stomach or duodenum, the endoscope allows visualization of the pancreas and adjacent structures.
There are two types of EUS scopes: radial scanning and linear array. The radial type scans in a plane perpendicular to the axis of the scope to produce 360˚ images similar to a CT “slice.” The transducer appears as a “bull’s-eye” within the image. The linear array type scans in a plane parallel to the axis of the scope. It is therapeutic in nature. It has the advantage of allowing visualization of a needle while performing a procedure.
Radial EUS
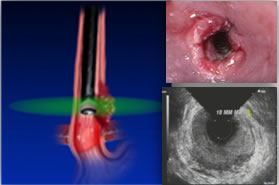
Radial EUS is used for staging only because it doesn’t have a working channel. One of the classic EUS images is esophageal cancer and its relationship with the esophageal wall. In the radial images, the esophageal wall is seen in cross section as a hypoechoic ring. Radial scopes produce an image similar to a CT scan, which is easier for most physicians to read because they are familiar with the imaging. Optimal imaging of the gastrointestinal wall is essential to determine the wall layer(s) involved by the disease process. To achieve optimal imaging and limit artifact, the sonographic plane must be placed as close as possible to being perpendicular to the intestinal wall.
Ultrasound images of organs contiguous to the gastrointestinal tract each have their own characteristic appearance. This includes large vessels and lymph nodes. Malignant lymph nodes will generally appear hypoechoic and round, with sharp margins. Benign lymph nodes appear irregular in shape, have indistinct margins and have mixed internal echoic features.
Linear EUS
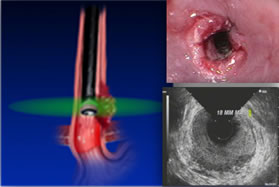
In the linear array images, one wall of the esophagus is seen and the hypoechoic linear structure is parallel to the axis of the scope.
A needle can be passed through the accessory channel of the endoscope and visualized in real time as it is directed through the esophageal wall and into lesions.
The choice between radial and linear scopes tends to be personal, based on the doctor’s training. For some time, radial scopes were the only option so most doctors trained with them, although they may switch to a linear scope if intervention of FNA is needed. Linear scopes were introduced later, to allow therapeutic devices, so physicians who trained on linear scopes tend to start with them.
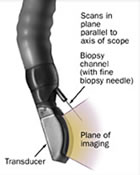
EUS-guided fine-needle aspiration (FNA) is performed in many large medical centers as part of routine esophageal cancer staging. The main clinical utility of EUS-FNA is cytological diagnosis of regional and distant tumor metastasis, which can diagnose tumor stage and influence treatment decisions.
The goal of FNA is to obtain cytological evidence of cancer (or confirm non presence of cancer cells) through tissue or fluid acquisition to diagnose and stage the extent of GI diseases. A fundamental principle of EUS-FNA is that the information obtained should have the potential to affect patient management. In addition, the indications for EUS-FNA should be guided by its diagnostic accuracy, cost effectiveness, patient comfort and safety. Using a linear scope which has a working channel, needles can be used to shrink cysts, sample tumors and take biopsies.
Contraindications/Challenges of FNA
EUS-FNA can acquire the diagnostic cells and tissue sample by puncturing a target organ through the digestive tract wall. Contraindications for EUS-FNA include “situations in which the FNA result would not affect management, inability to clearly visualize a lesion, a tumor mass or vessel interposed between the needle-to-target path, bleeding diathesis and risk of tumor seeding.” However, the advantages of doing an EUS/FNA is that it:
- Aids in the assistance in the staging and tissue acquisition of malignant disease through the EUS-guided FNA
- Aids in the assessment of benign disease
- Aids with interventional applications such as celiac plexus block or neurolysis and pseudocyst drainage
Specimen Handling of FNA
When solid tissue is sampled, a small amount of material accumulates in the hub of the needle. This material is immediately and gently expelled onto a clean glass slide, spread in a thin layer and rapidly dried by waving the slide in the air or by placing it in front of a fan or portable hair dryer. This is called making an “air-dried smear.” The slide is then stained with special dyes and examined under the microscope.
When a fluid sample is collected, air-dried smears are often prepared directly from the material in the syringe, and the remaining fluid is placed in transport tubes or containers. The smears and the containers are then sent to the laboratory for further analysis. This typically includes measurement of the cellularity and protein content of the fluid, as well as preparation of additional slides. If the sample is very thin and watery, sometimes the sample is concentrated before the slides are made, which provides more cells to look at. The slides are then stained with special dyes and examined for carcinoembryonic antigen (CEA), amylase levels and cytology.
Fine Needle Biopsy (FNB) is obtaining histological evidence of cancer through tissue or fluid to diagnose and stage the extent of GI diseases. The goal of FNB is to obtain evidence of cancer through core tissue samples, allowing for a more representative sample of the suspect lesion. Core biopsies may allow for additional testing or stains needed to verify specific, hard to diagnose disease states.
Although the diagnostic yield of EUS-FNA for pancreatic masses remains high, it continues to be suboptimal for non-pancreatic lesions. Technical limitations include availability of cytologic expertise, scant cellularity of specimens and inability to demonstrate lesion morphology and architecture. Histologic specimens or core biopsies may:
- improve assessment of tissue architecture,
- provide a more representative sample of the lesion,
- allow for immunohistochemistry or vital stains and
- potentially eliminate the need for OCE (On-site Cytopathology Evaluation), resulting in significant cost savings.
Specimen Handling of FNB

Specimen handling of FNB is critical. The appropriate handling of a core specimen is essential to ensure optimal diagnostic yield. If OCE (On-site Cytological Evaluation) is NOT used in the room, the specimen should be expelled with air, stylet or water flush directly into 10% formalin.
If the tissue acquired is a visible core, standard touch preparation is used. The core is slowly and carefully touched to the slide and then placed into a specimen container with formalin, as seen in the first photo.

In the event that only fragmented or scant tissue is obtained, the tissue is placed on a slide and a second slide should be used to gently crush the tissue and prepare an air-dried crush preparation. Any residual tissue should be fixed in 10% formalin for subsequent hematoxylin and eosin staining.
Pathologists look at tissue samples two different ways: FNA or FNB. FNA shows tissue samples at the cellular level, with slides prepared in Cytolyt. When using FNB, the sample is that of an intact core sample, revealing the true architecture of the tissue, which is preserved in Formalin. FNB does not improve the diagnostic yield of malignancy compared with FNA. However, the ability to obtain histologic specimens can aid in diagnosing benign conditions such as autoimmune or chronic pancreatitis, in which the assessment of tissue architecture is necessary to achieve a diagnosis. In addition, EUS-FNB should be considered in lesions with prior non-diagnostic EUS.
Compare the slides showing the differences between FNA and FNB from a pathologist’s perspective. The sample came from a 65-year-old man with a pancreatic mass in the head of the pancreas with EUS. EUS showed a well-defined round-shaped and slightly hypoechoic solid tumor in the uncinate. An FNB of the lesion was performed with a 25-gauge core biopsy needle.
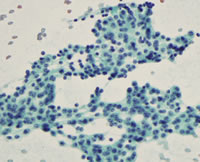
FNA-cellular level; cytological analysis
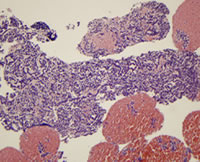
FNB-architecture of cells; histological analysis
- The specimen was expelled onto a glass slide. Macroscopic evaluation showed visible core, measuring 40 mm in length.
- The macroscopic visible core was picked up and placed into a formalin bottle for histologic analysis.
The remaining specimen was submitted for cytologic analysis. Cytologic and histologic findings showed neuroendocrine tumor.
Now let’s take a deeper look at why EUS/FNA would be needed for a patient and how the doctor would choose a system. It is best to look at FNA in two different areas: masses and cysts. With a mass or submucosal tumor, we are looking for tissue for diagnosis; with a cyst, we are looking for aspiration, either for evaluation or aspiration alone.
| Masses | Cysts |
|---|---|
|
|
Submucosal Tumors: Esophagus
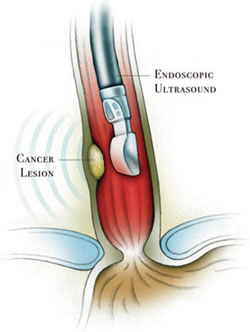
EUS-FNA in the esophagus is about staging. Imaging gives the doctor a sense of the depth of the tumor invasion. FNA will help validate the diagnosis. Not every EUS in the esophagus will involve FNA. Typically, if it is limited submucosa, doctors won’t do FNA and may go to EMR. A CT scan will often show if there is lymph node involvement, but a doctor may see something suspicious in the lymph nodes on EUS and decide to use FNA on the nodes.
It is important to know that a doctor should never use the same needle for FNA of the nodes that they used for FNA of the tumor itself. So they will always FNA the nodes first, then the tumor. The rationale for this order is the serious concern for the adverse event of tumor seeding where cells from a cancerous mass are passed to another mass at another location via the contaminated needle. Some physicians may want to use a smaller needle for the nodes, and a larger needle for the tumor. Being able to have a catheter in place and the ability to rapidly move needles in and out is efficient.
Submucosal Tumors: Stomach
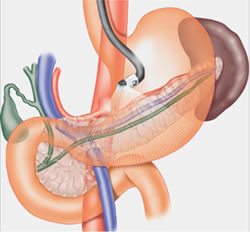
Gastrointestinal stromal tumors (GIST) are soft tissue sarcomas that can be located in any part of the digestive system, most commonly in the stomach and small intestine. The goal of EUS/FNA is to determine the level of tumor invasion into the wall of the stomach, and to acquire tissue to confirm diagnosis. While there are no recommendations on the number of passes necessary here, certainly the more tissue, the better.
Ideally, a pathologist should be present during the procedure. If the sample is too small, the pathologist would immediately inform the doctor, who in turn would switch to a larger needle. The pathologist may notice that there is too much blood in the sample, in which case the doctor would switch to a smaller needle and use less suction. Additional immunohistochemical staining of suspected GISTs is important for a definitive diagnosis. The adequacy and diagnostic yield of EUS-FNA specimens may be lower for submucosal lesions than those from pancreatic lesions and lymph nodes.
Pancreatobiliary Masses
Retrieving samples from pancreatobiliary masses can be complicated. The torqued scope position makes needle exchanges difficult and forcing a needle through a torqued channel risks scope damage. Repositioning of the scope may be necessary to allow needle exchanges. Typically, it takes about 5-7 passes of the needle to achieve adequate tissue diagnosis.
The physician will position the scope in the third part of the duodenum to obtain images and create the angles necessary to sample the target lesions. It can be challenging to maneuver through the pylorus with a side viewing scope. Remember, you are looking out the side of the scope and trying to move through a sphincter that is straight ahead in your field of vision. Working with the scope in the 3rd section of the duodenum creates torque on the scope, which compromises the integrity of the scope channel. All those twists and bends will flatten the channel, much like crimping a straw. Positioning of the scope is critical to retrieving a good sample and once proper positioning is achieved, the sample is taken.
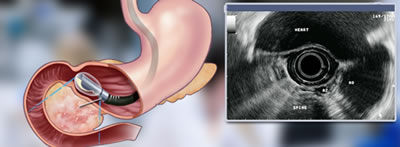
Typically, after the first sample is taken, a couple things happen. 1. The needle system is withdrawn and reinserted. 2. The scope will need to be repositioned in the stomach to create the straight channel.. 3. Once the needle is removed, the tissue is expelled. 4. The needle is reloaded through the channel and the physician will maneuver back into the duodenum. Fortunately, there are some systems that will allow you to leave the catheter in place and only remove and exchange the needle in a rapid exchange process, not losing time or positioning.
FNB in Solid Pancreatic Lesions
The ability to detect malignancy is reduced in patients with underlying pancreatitis because of the difficulty identifying a discrete mass lesion in this setting. FNB does not improve the diagnostic yield of malignancy compared with FNA. However, the ability to obtain histologic specimens can aid in diagnosing benign conditions such as autoimmune or chronic pancreatitis, in which the assessment of tissue architecture is necessary to achieve a diagnosis. EUS-FNB should be considered in lesions with prior nondiagnostic EUS.
Now let’s take a deeper look at cysts. With a cyst, we are looking for aspiration, either for evaluation or aspiration alone. With cysts, aspiration might be the only objective. In those cases, the doctor will typically only need one pass. But often times, the secretions from a cyst might be very mucinous. The process is typically started with a smaller needle because there is always concern about bleeding when using a larger needle. If the small needle is too small, substitution for a larger needle may be necessary. If multiple needles are needed, a scope which allows the physician to leave the catheter in place and exchange needles in a rapid method would be more efficient.
Scope position is relevant in pancreatic cyst cases. Doing an EUS + CFA [Cyst Fluid Analysis] aids in the differentiation between mucinous and non-mucinous cysts and the identification of specific subtypes and high-risk stigmata for surgical evaluation.
There are several products in the market for doctors to choose from. They need to explore their options and find the product which works best for them.
We know that patient teaching is important for a successful outcome. Let’s take a look at a few patient teaching considerations.
What Happens During an EUS?
During an EUS, the patient lies on his/her side while an endoscope with a small ultrasound probe built into the tip is passed through the patient’s mouth, the stomach and into the top part of the small intestine called the duodenum. Once in place, the ultrasound probe uses sound waves to create images of the pancreas and surrounding structures. Usually the EUS procedure will last 30-60 minutes, depending on additional treatments.Most patients receive IV anesthesia (Propofol); they are asleep and comfortable during the procedure.
What Should a Patient Do to Prepare for an EUS?
The healthcare team will give the patient exact instructions before an EUS procedure.The patient will be informed to remain NPO for six to eight hours prior to the procedure. Some patients receive antibiotics before the procedure to avoid infection. The doctor will inform the patient whether to continue taking these or other medications before the EUS procedure. EUS is usually performed under IV anesthesia (Propofol). Since the patient may feel drowsy after the procedure, arrangements should be made by the patient in advance to have someone else drive him/her home.
What is Expected After an EUS?
After the EUS procedure is completed, the patient will remain in the recovery room until the sedative medication has worn off. Since CO2 instead of room air is used almost routinely for insufflation during these procedures, the patient does not usually experience a feeling of fullness or the need to pass gas after the procedure. Also, the patient may experience changes in the bowel habits (for example, soft stool) after the procedure. The health care team will provide instructions regarding medications, eating, drinking and driving.
What Complications Can Occur with an EUS?
Complications are very rare, but they include infection of a pancreatic cyst, pancreatitis, gastrointestinal bleeding, perforation from the endoscope and reactions to anesthesia medications.
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) has been integral in transforming endoscopic GI practice from just an imaging model to more of an interventional tool. Beginning with the initial reports of EUS-FNA use in 1992, it has been part of a protocol ever since, in diagnosing and staging algorithms for evaluating benign and malignant diseases of the Gl tract (and adjacent organs). EUS-FNA has been seen as a major breakthrough in technology in its ability to provide definitive evidence of a malignancy, by being less subjective and more objective in providing a diagnosis.
CNE Certificate Process
Note: Your computer should be connected to a printer before completing the Post-Testing in order to permit printing your course certificate.

